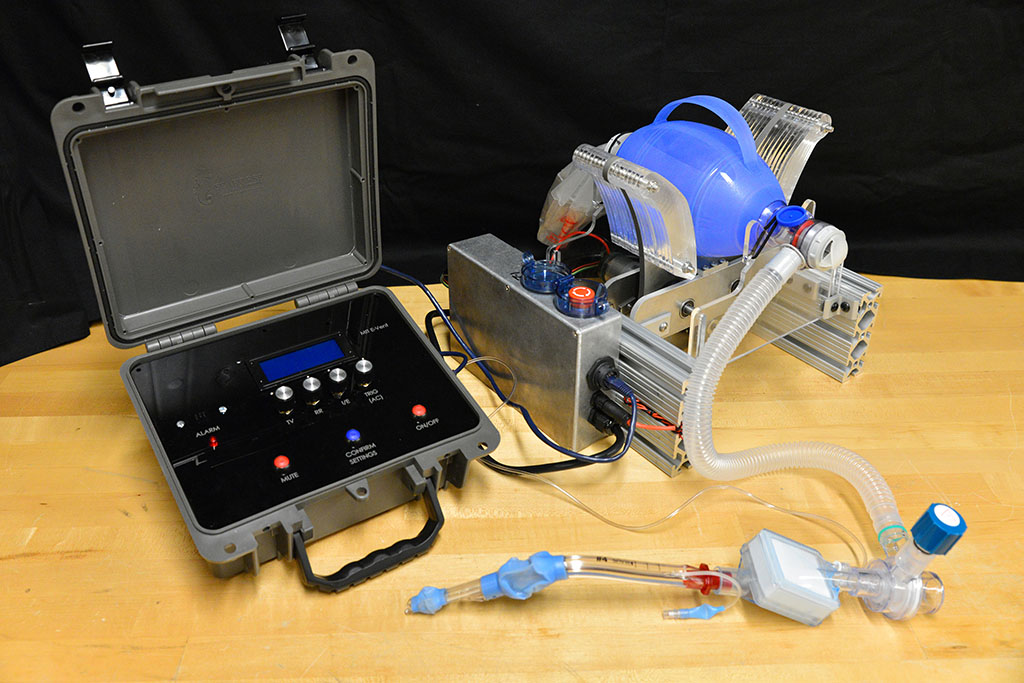
MIT team races to fill Covid-19-related ventilator shortage
It was clear early on in the unfolding Covid-19 pandemic that a critical need in the coming weeks and months would be for ventilators, the potentially life-saving devices that keep air flowing into a patient whose ability to breathe is failing.
Seeing a potential shortfall of hundreds of thousands of such units, professor of mechanical engineering Alex Slocum Sr. and other engineers at MIT swung into action, rapidly pulling together a team of volunteers with expertise in mechanical design, electronics, and controls, and a team of doctors with clinical experience in treating respiratory conditions. They started working together nonstop to develop an inexpensive alternative and share what they learned along the way. The goal was a design that could be produced quickly enough, potentially worldwide, to make a real difference in the immediate crisis.
In a very short time, they succeeded.
Just over a month since the team convened, production of the first devices based directly on its work has begun in New York City. A group including 10XBeta, Boyce Technologies, and Newlab has begun production of a version called Spiro Wave, in close collaboration with the MIT team. The consortium expects to quickly deliver hundreds of units to meet the immediate needs of hospitals in New York and, eventually, other hospitals around the country.
Meanwhile, the team, called MIT E-Vent, has continued their research to develop the design further. The next iteration will be more compact, have a slightly different drive system, and add a key respiratory function. Their overarching goal is to focus on safety and straightforward functionality and fabrication. 10XBeta, in both New York and Johannesburg, along with Vecna Technologies and NN Life Sciences in the Boston Area, are participating in this effort. 10XBeta was founded by MIT alumnus Marcel Botha SM ’06.
A complex design challenge
Alexander Slocum Jr. SB ’08, SM ’10, PhD ’13, a mechanical engineer who is now a surgical resident at the Medical College of Wisconsin, worked closely with his father, Slocum Sr., and MIT research scientist Nevan Hanumara MS ’06, PhD ’12 to help lead the initial ramp up.
“The numbers are frightening, to put it bluntly,” Slocum Jr. says. “This project started around the time of news reports from Italy describing ventilators being rationed due to shortages, and available data at that time suggested about 10 percent of Covid patients would require an ICU.” One of his first tasks was to estimate the potential ventilator shortage, using resources like the CDC’s Pandemic Response Plan, and literature on critical care resource utilization. “We estimated a shortage of around 100,000 to 200,000 ventilators was possible by April or May,” he says.
Hanumara, who is one of the project leads for the E-Vent team, says the team intends to offer open-source guidelines, rather than detailed plans or kits, that will serve as resources to enable skilled teams around the country and world — such as hospital-based engineering groups, biomedical device manufacturing companies, and industry groups — to develop their own specific versions, taking into account local supply chains.
“There's a reason we don't have a single exact plan [on the website],” Hanumara says. “We have information and reference designs, because this isn't something a home hobbyist should be making. We want to emphasize that it’s not trivial to create a system that can provide ventilation safely.”
“We saw all these designs being posted online, which is awesome that so many people wanted to help,” says Slocum Jr. “We thought the best first step would be to identify the minimum clinical functional requirements for safe ventilation, compare that to reported methods for managing ventilated patients with Covid, and use that to help us choose a design.”
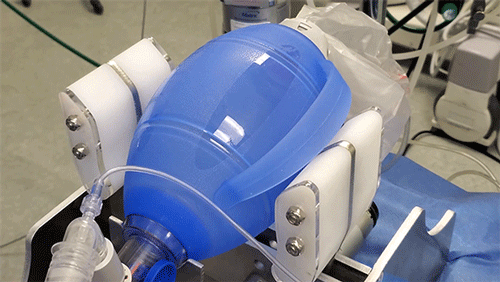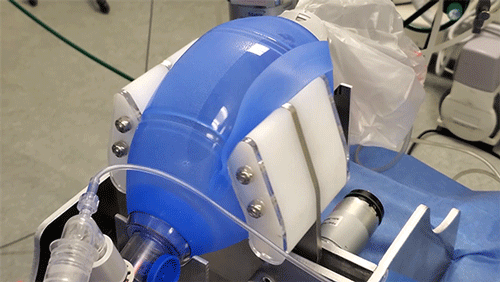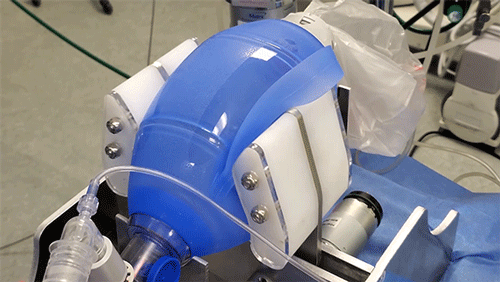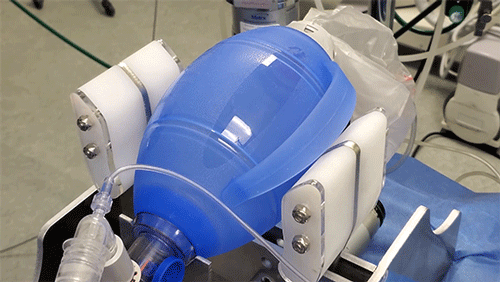
The principle behind the existing device is certainly simple enough: Take an emergency resuscitator bag (Ambu is a common brand), which hospitals already have in large numbers and which is designed to be squeezed by hand. Automating the squeezing — using a pair of curved paddles driven by a motor — would allow rapid scale-up. But there’s a lot more to it, Hanumara says: “The controls are really tricky, and they have required many iterations as our understanding of the clinical and safety challenge grew.”
Slocum Jr. adds, “Covid patients often require ventilation for a week or more, and in longer cases that would mean about a million breaths. The paddles are specifically designed to encourage rolling contact in order to minimize wear on the bag.”
The starting point was a design developed a decade ago as a student team project in MIT class 2.75 (Medical Device Design), taught by Slocum Sr. and Hanumara. The team’s paper gave the new project a significant head start in tackling the design problem now, as they make rapid progress in close consultation with clinical practitioners.
That integral involvement of clinicians “is one key difference between us and a lot of the others” working on this engineering problem, says Kimberly Jung, an MIT master’s student in mechanical engineering.
Jung — who previously served five years in the U.S. Army, earned an MBA at Harvard University, and started a spice business that is currently the largest employer of women in Afghanistan — has been acting as the team’s executive officer as well as part of the engineering team. She says “there's a lot of individuals and many small companies who are trying to make solutions for low-cost ventilators. The problem is that they just haven't adhered to clinical guidelines, such as the tidal volume, inspiration-to-expiration ratio, breath per minute rate, maximum pressures, and key monitoring for safety. Developing these clinical requirements and translating them into engineering design requirements takes a lot of time and effort. This is a year-long research and development process that has been condensed into several weeks.”
A team assembles
Others got pulled into the team as the project ramped up. Coby Unger, an industrial designer and instructor at the MIT Hobby Shop, started building the first prototypes in the machine shop. Jung recruited her classmate and neighbor, Shakti Shaligram SM ’19, to help with machining, and also brought in Michael Detienne, an electrical engineer and member of the MITERS makerspace. Two students at the MIT Maker Workshop helped with initial fabrication with stock borrowed from the MIT Laboratory for Manufacturing and Productivity shop. Looking for pressure sensors, Hanumara reached out to David Hagan PhD ’20, CEO of an MIT spinoff company called QuantAQ, and he joined the team. The website was rapidly deployed by Eric Norman, a communications expert who had worked with Hanumara on another MIT project.
Realizing that feedback and control systems were crucial to the device’s safe operation, the team early on decided they needed help from specialists in that area. Daniela Rus, head of MIT’s Computer Science and Artificial Intelligence Laboratory, suggested research scientist Murad Abu-Kalaf and graduate students Teddy Ort and Brandon Araki, who all eagerly agreed to join the volunteer team. Ort’s roommate, Amado Antonini SM ’18, also joined the team to assist with motor controls.
Meanwhile, alumnus Albert Kwon SB ’08, HST ’13, an anesthesiologist at Westchester Medical Center and assistant professor of anesthesiology at New York Medical College, was recruited by Slocum Jr. to join the project early on. Kwon was granted leave from his job at Westchester to devote time to the project, providing clinical guidance on the kinds of controls and safety systems needed for the device to work safely. “Westchester Medical Center gave him up, which is very special, and he's been working to translate the technical to clinical, and explain the scenarios that fit with a stripped-down system like this,” Hanumara says. Jay Conor, a surgeon at Mt. Auburn Hospital and part of the Medical Device Design course teaching team, Christoph Nabzdyk, a cardiothoracic anesthesiologist and critical care physician at Mayo Clinic and long-time colleague of Kwon, and Dirk Varelmann, another anesthesiologist from Brigham and Women’s Hospital, and many other clinicians advised the MIT E-Vent team.
A spark to help others fill the gap
“While our design cannot replace a full featured ventilator,” Hanumara stresses, “it does provide key ventilation functions that will allow health care facilities under pressure to better ration their ICU ventilators and human resources, in a bad scenario.”
In a way, he says, “we're turning the clock back, going back to the core parameters of ventilation.” Before today’s electronic sensors and controls were available, “doctors were trained to adjust the ventilators based on looking directly at physiological responses of the patient. So, we know that’s doable. … The patient himself is a reasonable sensor.”
While the federal government has now established contracts with large manufacturing companies to start producing ventilators to help meet the urgent need, that process will take time, Jung says, leaving a significant gap for something to meet the need in the meantime. “The fastest these large manufacturers can spin up is about two months,” she says.
“This need will probably be even more pronounced in the emerging markets,” Hanumara adds.
The team doesn’t plan to directly launch their own production, or even to provide a single, detailed set of plans. “Our goal is to put out a really solid reference design,” Hanumara says “and to a limited extent help big groups scale it. We have shared great learnings with our local industry collaborators.” It will be up to local teams to adapt the design to the materials and parts that they can reliably obtain and the particular needs of their hospitals.
He says “your mechanical and electrical engineering team will have to inquire as to what's in their supply chain and what fabrication methods they have easily available to them and adapt the design. The base designs are intended to be really adaptable, but it may require modifications. What motors can they source? What motor drivers and controllers do electrical team need to look at? What level of controls and safeties do their clinicians require for their patient population and how should this be reflected in the code? So, we can't put out an exact kit,” says Hanumara.
The hope is to provide a spark to start teams everywhere to further develop and adapt the concept, Hanumara says. “Provided clinical safety is shown, we'll probably see many of these around the world, with some shared DNA from us, as well as local flavors. And I think that will be beautiful, because it will mean that people all over are working hard to help their communities.”
“I'm super proud of the team,” Jung says, “for how each of us has stepped up to the plate and stuck with it despite the internal and external challenges. All of us have one mission in mind, which is to save lives, and that's what has kept us together and turned us into a quirky MIT family.”