
Professor
Ioannis Yannas
Professor of Polymer Science & Engineering
Interests
- Basic science of organ regeneration
- Regeneration of skin, peripheral nerves, spinal cord and conjunctiva
- Molécular biological mechanism of induced regeneration
Professor Yannas News + Media
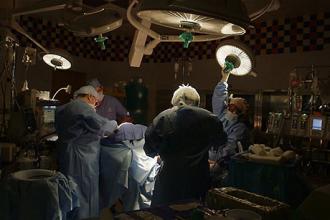
Hope Regenerated: A life-saving discovery at MechE
A “failed experiment” became a life-saving discovery by MIT Professor Ioannis V. Yannas and his colleague Dr. John Burke when their search for a better way to treat severe burn victims led to the discovery of organ regeneration.
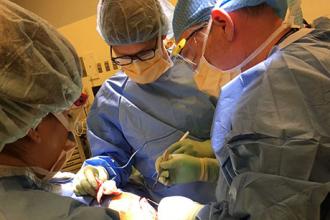
Using mechanical forces to improve wound healing
Alum Dennis Orgill SM ’80, PhD ’83 applies the mechanical engineering principles he learned from Professor Ioannis V. Yannas to the operating room.

Ioannis Yannas to be Inducted into the National Inventors Hall of Fame
Yannas’ invention of artificial skin did more than just block infection and retain moisture — it actually helped to regenerate the skin.
Faculty Details
Education
-
1957
HARVARD COLLEGE
A.B. -
1959
MASSACHUSETTS INSTITUTE OF TECHNOLOGY (MIT)
M.S. -
1965
PRINCETON UNIVERSITY
M.A. -
1966
PRINCETON UNIVERSITY
Ph.D.
Research Interests
The principal research interest of Dr Yannas is the process of induced organ regeneration used to replace organs that are either severely injured or are terminally diseased.
Initial discovery of dermis regeneration. In 1976 Yannas and John F. Burke, MD discovered the first scaffold with regenerative activity. Although the strctural features of a scaffold with regenerative activity were not appreciated at that time, they were eventually (1989, 2015; see references below) recognized as those of a highly porous analog of the extracellular matrix based on type I collagen, a biodegradable scaffold with highly specific structural features. These required features included a specific range of the pore size, defined degradation half-life and specified surface chemistry. When this cell-free scaffold was grafted on deep skin wounds in guinea pigs it was unexpectedly observed in 1976 that it led to strong delay of wound contraction and eventually to wound closure by formation of scarless tissue that had the appearance of dermis. The full significance of this discovery was not understood at that time; it was explained adequately about 40 years later (see below). Use of this scaffold, named dermis regeneration template (DRT), with full-thickness skin wounds in animal and humans led to synthesis of a nearly physiological dermis (1975-81). When this scaffold was seeded with keratinocytes it led to simultaneous regeneration of the dermis and the epidermis in animals and in humans (Orgill PhD thesis, 1981-83). [Early versions of the active scaffold were based on a graft copolymer of type I collagen and chondroitin 6-sulfate, a glycosaminoglycan.] An account of the early discovery of a process for dermis regeneration has been published (Yannas IV. Hesitant steps from the artificial skin to organ regeneration. Regenerative Biomaterials 5: 189-195 (2018)).
This outcome was totally unexpected: Although the epidermis regenerates spontaneously on a pre-existing dermal substrate, the completely excised dermis does not regenerate spontaneously in the adult mammal. The work eventually led to development of a medical device (commercial name: IntegraTM) that is currently used with increasing frequency to treat patients who have lost skin due to trauma, or are undergoing plastic surgery, or with patients suffering from chronic skin wounds. Its use in skin regeneration has been documented in over 300 clinical studies over the past several years, many of which are cited in the following website: http://www.ncbi.nlm.nih.gov/pubmed/?term=Integra+substitute+skin. The collagen scaffold work by the MIT group during the period 1976-1990 has provided the original paradigm in the fields of regenerative medicine and tissue engineering. This work resulted in the first patent on induced organ regeneration. (Yannas, I.V., J.F. Burke, D.P. Orgill, and E.M. Skrabut, Method of Promoting the Regeneration of Tissue at a Wound, U.S. Pat. 4,418,691, December 6, 1983).
From skin to peripheral nerves. The work with skin has been extended by Yannas and coworkers at MIT to regenerate peripheral nerves. Early work showed that the transected rat sciatic nerve was regenerated over unprecedented distances using, at first, silicone tubes filled with DRT and later simply a tubular connector based on DRT. These findings were used in an industrial laboratory to develop a commercial device (NeuragenTM) which is currently used to treat humans with paralysis of extremities resulting from severe injury (Orgill, MD thesis, 1985; L. Chamberlain PhD thesis, 1998-2000; E. Soller, PhD thesis, 2010-12). It was also used to regenerate the conjunctiva in adult rabbits (Hsu et al., 2000).
Study of the wound healing process in skin and peripheral nerves by the Yannas group showed that both organs healed by contraction and scar formation. In healing wounds, contraction is mediated by myofibroblasts (MFB), which appear to apply a planar mechanical stress field in full-thickness skin wounds and a circumferential mechanical compression field around the stump of a transected nerve. These mechanical fields have accounted well for the observed orientation of MFB axes in healing wounds in the two organs as well as the eventual orientation of collagen fibers both in dermal scar (planar orientation) and neural scar (circumferential orientation). The difference between the spatial features of wound contraction in the two organs appears to be entirely a result of differences in the macroscopic geometry of the respective organ, not due to differences in the cell biology of wound healing in each organ. This conclusion suggests the possibility that other organs which share the same wound healing process as skin and peripheral nerves may be successfully subjected to the same regenerative treatment based on use of DRT. It was eventually realized by the MIT group (2014-2016) that the entire regenerative treatment in these two organs amounted to a simple modification of the normal wound healing process. It was concluded that what was preventing the normal wound healing process from inducing regeneration (rather than normal healing by wound contraction and scar formation) was a scaffold with the appropriate surface chemistry, which would induce appropriate surface biological processes leading to blocking of wound contraction. Normal wound contraction had, therefore, to be blocked in order to induce regeneration; there was no apparent need to apply exogenous cells or active soluble substances, such as stem cells or growth factors. This conclusion simplifies greatly the methodology of organ regeneration.
Regeneration of the crushed spinal cord in animals. Our findings (in collabaration with coworkers in Crete, Greece) show that injured mice treated with neural stem cell (NSC)-seeded scaffolds performed significantly better than the untreated crush group on the ladder-walking test over 9 weeks post injury. Histological assessment of spinal cord sections revealed that NSC-seeded scaffolds managed to stay in place and protect seeded cells in vivo for at least 9 weeks, significantly increased axonal regeneration, and significantly reduced astrogliosis. (Alexandra Kourgiantaki, Dimitrios S. Tzeranis, Kanelina Karali, Sotirios Psilodimitrakopoulos, Maria Nikou, Ioannis V. Yannas, Emmanuel Stratakis, Kyriaki Sidiropoulou, Ioannis Charalampopoulos, and Achille Gravanis (2020). Neural stem cell delivery via porous collagen scaffolds promotes neuronal differentiation and locomotion recovery in spinal cord injury. npjRegenerative Medicine. 5, 1-14).
Mechanism of regeneration. The molecular-biological mechanism of scaffold regenerative activity was elucidated by the same MIT group (Yannas, Tzeranis, So, 2007-2014). The regenerative activity of the DRT scaffold appears to be related closely to the known contraction blocking property of DRT. Blocking of wound contraction occurs only when the structure of the collagen scaffold has been modified to provide critical levels of the average pore diameter (20-120 µm), degradation half-life (14 ± 7 days) and required features for a specific surface chemistry. Critical features of the surface chemistry include a minimal level of ligand density for two integrins, α1β1 or α2β1, that exceeds approximately 200 μΜ α1β1 or α2β1 μΜ ligands (Tzeranis, PhD thesis, 2013-2015). Binding of a sufficient number of MFB to the ligands GFOGER and GLOGEN on the scaffold surface, during a critical period after the wound is generated, appears to explain the drastic modification of the MFB phenotype which is observed when DRT is grafted in the wound. The MFB phenotype changes due to DRT are observed as simultaneous reduction in MFB density in the wound, dispersion of MFB assemblies and disorientation of MFB long axes. This profound phenotype modification of contractile fibroblasts in the presence of DRT appears to explain the cancellation of macroscopic contraction force, an event which ushers in regeneration. A summary of the mechanism of organ regeneration at differnt scales has been poublished (Yannas IV, Tzeranis DS, So PTC. (2018) Regeneration mechanism for skin and peripheral nerves clarified at the organ and molecular scales. Current Opinion in Biomedical Engineering, 6:1-7)
Severely injured vs diseased organs. It has been shown in numerous clinical studies that diseased skin, beset with pathology unrelated to trauma, can be regenerated provided that the diseased region of the organ has been reduced to a viable wound prior to grafting with DRT. In this clinical approach, deliberate surgical injury is inflicted in order to initiate the wound healing process, which is then modified using DRT in order to induce regeneration. For example, with skin that had become diseased by skin necrosis due to purpura fulminans (Besner and Klamar, 1998), use of DRT following skin excision led to dermis regeneration. These independent findings suggest that an organ which has become severely dysfunctional can be replaced by deliberating generating a wound in the organ, followed by grafting with DRT to induce regeenration of a functional tissue mass.
The methodology for organ regeneration described by the Yannas group appears to be applicable to organs other than skin and peripheral nerves or the conjunctiva. The necessary conditions for inducing regeenration appear to be availability of a severe, though normal, wound and treatment of that wound by a scaffold with regenerative activity. Applicability to other organs appears to require that a wound in the candidate organ heals by the same mechanism as in skin, peripheral nerves and the conjunctiva. The induced regeneration approach using an active scaffold is increasingly used as an alternative to currently popular clinical treatment of skin loss by autografting, suggesting that it may also be a viable alternative to transplantation of other organs requiring replacement. However, this method is probably not directly applicable to wounds in organs, e.g., bone, where wounds heal by processes not directly related to those in organs that have been shown capable of induced regeenration.
Description of organ regeneration methods in a monograph. Detailed references to the anatomical structures involved in regeneration and to the mechanism of regenerative activity are described in Yannas’ book Tissue and Organ Regeneration in Adults, now in its second edition (NY: Springer, 2015).
The story of a severely burned patient who was treated with IntegraTM was authored by Michael McCarthy in the book, The Sun Farmer (Chicago: Ivan R. Dee, 2007).
A 10-minute MIT video describing the clinical application of induced skin regeneration can be viewed here: A Lifesaving Discovery at MIT. In the early 1980s, before confirmation became available that the collagen scaffold induces regeneration of skin, the scaffold was commonly referred to as "artificial skin".
Honors for discoveries. For his discoveries in organ regeneration Yannas was elected to the US National Academy of Medicine (1987) and the National Academy of Engineering (2017), and was inducted in the US National Inventors Hall of Fame (2015). Other awards were also received.
Bio
Biographical information. Ioannis Yannas was born in Athens, Greece.
In 1953 Ioannis entered Harvard College and majored in Chemistry, graduating in 1957 with the A.B. degree. He then entered MIT where he earned a M.S. degree in Chemical Engineering Practice in 1959. After a period of industrial research on polymers at Grace Co., Cambridge, MA, he entered Princeton University where he earned the M.A. degree (1965) and the Ph.D degree (1966), both in Physical Chemistry. His doctoral thesis dealt with the viscoelastic behavior and thermal transitions in gelatin, the amorphous counterpart of the protein, collagen.
Since 1966 Yannas has been employed as a faculty member by Massachusetts Institute of Technology (MIT), Cambridge, MA. He currently holds a full-time appointment in the Mechanical Engineering Department. He also holds an appointment at the Harvard-MIT Health Sciences and Technology Program. He teaches classes in Biomaterials-Tissue Interactions; Tissue Engineering and Organ Regeneration; and Design of Medical Devices and Implants.
Online Data
Early reports of regeneration of the dermis using the dermis regeneration template (DRT) by the MIT group in collaboration with J.F. Burke, MD, Harvard Medical School, appeared in the following:
- Yannas I.V. and J.F.Burke. 1980. Design of an artificial skin. I. Basic Design Principles. J Biomed Mater Research 14, 65-81.
- Yannas, I.V. 1981. Use of artificial skin in wound management. In The Surgical Wound, edited by Dineen, P. Philadelphia: Lea & Febiger.
- Yannas, I.V., J.F. Burke, M. Warpehoski, P. Stasikelis, E.M. Skrabut, D. Orgill, and D.J. Giard. 1981. Prompt, long-term functional replacement of skin. Trans. Am. Soc. Artif. Intern. Organs 27:19–22.
- Burke, J.F., I.V. Yannas, W.C.Q. Jr., C.C. Bondoc, and W.K. Jung. 1981. Successful use of a physiologically acceptable artificial skin in the treatment of extensive burn injury. Ann. Surg. 194:413–428.
- Yannas, I.V., J.F. Burke, D.P. Orgill, and E.M. Skrabut. 1982. Wound tissue can utilize a polymeric template to synthesize a functional extension of skin. Science 215:174–176.
- Yannas, I.V., J.F. Burke, D.P. Orgill, and E.M. Skrabut. 1982. Regeneration of skin following closure of deep wounds with a biodegradable template. Trans. Soc. Biomater. 5:24–27.
Confirmation of regeneration of the dermis following treatment of full-thickness (dermis-free) skin wounds in adult guinea pigs was first reported in the following publications based on studies by Dr George F. Murphy, Laboratory of Dermatopathology, Brigham and Womens Hospital, Boston, MA:
- Yannas, I.V., E. Lee, D.P. Orgill, E.M. Skrabut, and G.F. Murphy. 1989. Synthesis and characterization of a model extracellular matrix which induces partial regeneration of adult mammalian skin. Proc. Natl. Acad. Sci. USA 86:933–937.
- Murphy,G.F., D.P.Orgill, I.V.Yannas. 1990. Partial dermal regeneration is induced by biodegradable collagen-glycosaminoglycan grafts. Lab. Invest. 62:305–313.
Independent confirmation of regeneration of dermis was obtained by C.C. Compton, Department of Dermatology, Brigham and Women’s Hospital, Boston, MA.
- C.C. Compton, C.E. Butler, I.V. Yannas, G. Warland, and D.P. Orgill. 1998. Organized skin structure is regenerated in vivo from collagen-GAG matrices seeded with autologous keratinocytes. J. Invest. Dermatol. 110:908–916.
Structural identification and required structural features of the dermis regeneration template (DRT) were reported in:
- Yannas, I.V., E. Lee, D.P. Orgill, E.M. Skrabut, and G.F. Murphy. 1989. Synthesis and characterization of a model extracellular matrix which induces partial regeneration of adult mammalian skin. Proc. Natl. Acad. Sci. USA 86:933–937.
- Soller EC, Tzeranis DS, Miu K, So PT, Yannas IV. 2012. Common features of optimal collagen scaffolds that disrupt wound contraction and enhnce regeneration both in peripheral nerves and in skin. Biomaterials. 2012 Jun;33(19):4783-91.
- Yannas I.V., D. Tzeranis and P.T. So. Surface biology of collagen scaffold explains blocking of wound contraction and regeneration of skin and peripheral nerves. Biomedical Materials. 11:014106 (2015).
Early studies of peripheral nerve regeneration:
- Yannas, I.V., D.P. Orgill, J. Silver, T.V. Norregaard, N.T. Zervas, and W.C. Schoene. 1985. Polymeric template facilitates regeneration of sciatic nerve across 15 mm gap. Trans. Soc. Biomater. 8:146, 1985.
- Orgill D.P. 1985. Partial regeneration in mammalian tissues using polymeric materials. MD thesis, Harvard Medical School.
- Yannas, I.V., D.P. Orgill, J. Silver, T. Norregaard, N.T. Zervas, and W.C. Schoene. 1987. Regeneration of sciatic nerve across 15-mm gap by use of a polymeric template. In Advances in Biomedical Materials, edited by Gebelein, C.G. Washington, DC: American Chemical Society.
- Yannas, I.V., T.V. Norregaard, J. Silver, N.T. Zervas, J.F. Kirk, and M.J. Colt. 1987. Relations between properties of collagen-glycosaminoglycan graft and morphology of regenerating nerve. Part I. Trans. Soc. Biomater. 10:6.
- Yannas,I.V., A.S.Chang, C.Krarup, R.Sethi, T.V.Norregaard,and N.T.Zervas.1988. Conduction properties of peripheral nerves regenerated by use of copolymer matrices with different biodegradation rates. Soc. Neurosci. Abs. 14:165.
Advanced studies in peripheral nerve regeneration.
- Chamberlain, L.J., I.V. Yannas, H.-P. Hsu, G. Strichartz, and M. Spector. 1998. Collagen-GAG substrate enhances the quality of nerve regeneration through collagen tubes up to level of autograft. Exp. Neurol. 154:315–329.
- Chamberlain, L.J., I.V. Yannas, H.-P. Hsu, and M. Spector. 2000. Connective tissue response to tubular implants for peripheral nerve regeneration: The role of myofibroblasts. J. Comp. Neurol. 417:415–430.
- Chamberlain, L.J., I.V. Yannas, H.-P. Hsu, G.R. Strichartz, and M. Spector. 2000. Near terminus axonal structure and function following rat sciatic nerve regeneration through a collagen-GAG matrix in a 10-mm gap. J. Neurosci. Res. 60:666–677.
- Yannas IV, Zhang M, Spilker MH, 2007. Standardized criterion to analyze and directly compare various materials and models for peripheral nerve regeneration. J Biomater Sci Polymer Ed. 18(8):943-66.
- Soller EC, Tzeranis DS, Miu K, So PT, Yannas IV. 2012. Common features of optimal collagen scaffolds that disrupt wound contraction and enhnce regeneration both in peripheral nerves and in skin. Biomaterials. 2012 Jun;33(19):4783-91.
Mechanistic studies of organ regeneration
- Yannas, I.V. and J.F. Burke. 1980. Design of an artificial skin I. Basic design principles. J. Biomed. Mater. Res. 14:65–81.
- Yannas IV. 1990. Biologically active analogues of the extracellular matrix: Artificial skin and nerves. Angew. Chemie Intl. Ed. English 29:20-35.
- Yannas, I.V. 1997. Models of organ regeneration processes induced by templates. Ann. N. Y. Acad. Sci. 831:280–293.
- Yannas IV. 2005. Similarities and differences between induced organ regeneration in adults and early foetal regeneration. J Roy Soc Interface 2:403-417.
- Soller EC, Tzeranis DS, Miu K, So PT, Yannas IV. 2012. Common features of optimal collagen scaffolds that disrupt wound contraction and enhnce regeneration both in peripheral nerves and in skin. Biomaterials. 2012 Jun;33(19):4783-91. doi: 10.1016/j.biomaterials.2012.03.068. Epub 2012 Apr 6.
- I.V. Yannas, D. Tzeranis and P.T. So. Surface Biology of Collagen Scaffold Explains Blocking of Wound Contraction and Regeneration Of Skin and Peripheral Nerves. Biomedical Materials, 11:014106 (2015).
- Tzeranis DS, Soller EC, Buydash MC, So PT, Yannas IV. In situ quantification of surface chemistry in porous collagen biomaterials. Ann. Biomed. Eng. 44:803-15 (2016).
- Yannas IV, Tzeranis DS, So PTC. Regeneration of injured skin and peripheral nerves requires control of wound conraction, not scar formation. Wound Repair Regen. 25:177-191 (2017). doi: 10.1111/wrr.12516. Epub 2017 Apr 27
Honors + Awards
- 2017: National Academy of Engineering, elected Member “for codeveloping the first commercially reproducible artificial skin that facilitates new growth, saving the lives of thousands of burn victims”.
- 2015: National Inventors Hall of Fame. Inducted as Member based on U.S. Pat. 4,418,691 (December 6, 1983) awarded to IV Yannas, J.F. Burke, D.P. Orgill, and E.M. Skrabut, for “Method of Promoting the Regeneration of Tissue at a Wound". It is believed that this is the first patent describing organ regeneration.
- 2011: American Burn Association establishes Burke/Yannas annual bioengineering award
- 2007: Matrix Biology Group Lecturer, London
- 2002: Ray A. and Robert L. Kroc Lecturer, MIT
- 2002: Sophia Award, Greek Institute, Cambridge, MA
- 1996: Fellow in Biomaterials Science and Engineering (FBSE), Society for Biomaterials
- 1993: Founding Fellow, American Institute of Medical and Biological Engineering
- 1992: Clemson Award for Applied Science and Engineering, Society for Biomaterials
- 1988: Doolittle Award of the American Chemical Society
- 1987: National Academy of Medicine, elected member.
- 1985: Society of Plastics Engineers, Medical Plastics Division, "Best Technical Paper Award"
- 1982: Society for Biomaterials, Founders Award
- 1982: Society of Plastics Engineers, Fred O. Conley Award
- 1982: Cutty Sark/Science Digest Award
- 1982: Zinon Papanastassiou Memorial Lecturer, Hellenic College
- 1981: Technology Magazine, selected for inclusion among "The Technology 100"
- 1981: American Society for Artificial Internal Organs, selected among "Four Best Abstracts", Annual Meeting
- 1978: Hellenic Medical Society of New York, Annual Award
- 1977: Greek World Magazine, Annual Award
- 1968: DuPont Young Faculty Award, MIT
- 1963: Public Health Service Fellow, Princeton University
- 1958: Esso Standard Oil Fellow, MIT
- 1954: Harvard College Scholar, Harvard University
Memberships
- National Academy of Engineering
- National Academy of Medicine
- National Inventors Hall of Fame
- American Institute of Medical and Biological Engineering (Founding Fellow)
- American Society for Cell Biology
- Biomedical Engineering Society (Charter Member)
- American Chemical Society
- Society for Biomaterials (Fellow)
- American Society for Cell Biology
Professional Service
- 1972-Present: National Institutes of Health, grant reviewer
- 1966-Present: Several Journals, reviewer
- 1977, 2008: National Institute of Health, Study section, Member
- 1980: National Institute of Health Study section, Chairman
- 1982: National Institute of Health, Member, Consensus Panel, Conference on Biomaterials
- 1985-87: National Research Council, Committee of Advanced Structural Materials
- 1986-Present: Editorial Board: Journal of Biomedical Materials Research Part A
- 1990-2007: Editorial Board: Journal of Materials Science: Materials in Medicine
- 1995-2008: Editorial Board: Tissue Engineering (USA)
- 2005-2007: Editorial Board: J Royal Soc Interface
- 2005- Present: Editorial Board: Biomedical Materials (China)
- 2009: Editorial Board: J Tissue Engineering (UK)
- 2010-Present: Editorial Board: Materials Science and Engineering C: Materials for Biological Applications
MIT Service
Can be supplied upon request.
Teaching
Teaching Appointments at MIT
- 1957: Teaching Assistant - Industrial Chemistry
- 1958: Teaching Assistant - Chemical Engineering
- 1966-68: Assistant Professor, Department of Mechanical Engineering
- 1968-69: DuPont Assistant Professor, Department of Mechanical Engineering
- 1969-72: Associate Professor without tenure, Department of Mechanical Engineering
- 1973-78: Tenured Associate Professor, Department of Mechanical Engineering
- 1978-Present: Professor, Department of Mechanical Engineering
- 1983-2000: Professor, Materials Science and Engineering
- 2006-2012: Professor, Department of Biological Engineering
- 1978-2000: Professor, Harvard-MIT Program in Health Sciences and Technology, MIT
Publications
A. Books:
Sole author or editor
- Yannas IV, Tissue and Organ Regeneration in Adults. New York, Springer, 2001.
- Yannas IV, Tissue and Organ Regeneration in Adults. Extension of the Paradigm to Several Organs. Second Edition. New York, Springer, 2015.
- Editor, Regenerative Medicine. Two volumes. New York, Springer. 2005.
B. Papers in Refereed Journals
- 1. Yannas, I.V. (J.B.) and Gonzales, R.N., "A clear instance of rheopectic flow", Nature 191:1384-1385 (1961).
- 2. Yannas, I.V. (J.B.) and Gonzales, F.N., "Phenomenological Characterization of a Rheopectic Suspension", Rheological Bulletin 30, No. 2 (1961).
- 3. Yannas, I.V. (J.B.) and Gonzalez, R.N., "Low Shear Viscometry in the Prediction of Coating Performance", Technical Association of Pulp and Paper Industry Journal 45:156-159A (1962).
- 4. Yannas, I.V., (J.B.) "Fractionation of Chemically Heterogeneous Latex Particles by Centrifugation", Journal of Polymer Science A2:1633-1640 (1964).
- 5. Yannas, I.V., (J.B.) "Highly Precise Density Determination for Polymers in Latex Form", Polymer Letters 2:1005-1008 (1964).
- 6. Yannas, I.V. (J.B.) and Isgur, I.D., "Chemically Heterogeneous Populations of Copolymer Latex Particles. Preparation, Fractionation, and Characterization", Journal of Polymer Science A2:47194726 (1964).
- 7. Yannas, I.V. and Tobolsky, A.V., "Viscoelastic Properties of Plasticized Gelatin Films", Journal of Physical Chemistry 68:3880-3882 (1964).
- 8. Yannas, I.V. and Tobolsky, A.V., "Approximate Master Curves for Amorphous Polymers from Modulus-Temperature Data", Journal of Macromolecular Chemistry 1(2):399-402 (1966).
- 9. Yannas, I.V. and Tobolsky, A.V., "Transitions in Gelatin—Nonaqueous-diluent Systems", Journal of Macromolecular Chemistry 1(4):723-737 (1966).
- 10. Yannas, I.V. and Tobolsky, A.V., "Crosslinking of Gelatin by Dehydration", Nature 215:509-510 (1967).
- 11. Yannas, I.V. and Tobolsky, A.V., "Stress Relaxation of Anhydrous Gelatin Rubbers", Journal of Applied Polymer Science 12:1-8 (1968).
- 12. Yannas, I.V. and Tobolsky, A.V., "High-Temperature Transformations of Gelatin", European Polymer Journal 4:257-264 (1968).
- 13. Yannas, I.V., "Isochronal Temperature-Concentration Diagram for a Polymer-Diluent System", Journal of Polymer Science A2(6):687-694 (1968).
- 14. Yannas, I.V., "Vitrification Temperature of Water", Science 160:298-299 (1968).
- 15. Yannas, I.V., "Massive Internal Fracture of an Amorphous Polyester", Science 166:227-228 (1969).
- 16. Yannas, I.V., "Involvement of articular cartilage in a linear relaxation process during walking", Nature 227, 1358-1360 (1970).
- 17. Yannas, I.V. and Lunn, A.C., "The transition from linear to non-linear viscoelastic behavior, Part I, Creep of polycarbonate", Journal of Macromolecular Science B4:603-620 (1970).
- 18. Yannas, I.V. and Lunn A.C., "Infrared spectroscopic evidence for polycarbonate chain motion below Tg", Polymer Letters 9:611-615 (1971).
- 19. Yannas, I.V. and Haskell, V., "Utility of the Green-Rivlin theory in polymer mechanics", Journal of Applied Physics 42:610-613 (1971).
- 20. Yannas, I.V., Sung, N.-H., and Lunn, A.C., "The transition from linear to nonlinear viscoelastic behavior. Part II. Stress relaxation of polycarbonate", Journal of Macromolecular Science B5:487-503 (1971).
- 21. Yannas, I.V., "Collagen and gelatin in the solid state", Reviews in Macromolecular Chemistry C7:49-104B (1972).
- 22. Yannas, I.V., "The transition from linear to nonlinear viscoelastic behavior. Part III. Linearity below and above Tg", Journal of Macromolecular Science B6:91-100 (1972).
- 23. Yannas, I.V. and Doyle, M.J., "Comparison of optical and mechanical limits of linear relaxation behavior in glassy polycarbonate", Journal of Polymer Science A-2(10):159-170 (1972).
- 24. Yannas, I.V. and Huang, C., "Viscoelastic distinction between helical and coiled macromolecules", Macromolecules 5:99-100 (1972).
- 25. Yannas, I.V. and Huang, C., "Fracture of tendon collagen", Journal of Polymer Science A2 (10):577-584 (1972).
- 26. Lunn, A.C. and Yannas, I.V., "Chain-backbone motion in glassy polycarbonate studied by polarized infrared spectroscopy", Journal of Polymer Science A2(10):2189-2208 (1972).
- 27. Yannas, I.V. and Olson, D.A., "Linear relaxation analysis of the mechanochemical transformation of collagen fibers", Biopolymers 11:899-912 (1972).
- 28. Yannas, I.V., Sung, N-H. and Huang, C., "Resolution of components of the optical rotation tensor of collagen", Journal of Physical Chemistry 76:2935 (1972).
- 29. Yannas, I.V. and Grodzinsky, A.J., "Electromechanical energy conversion with collagen fibers in an aqueous medium", Journal of Mechanochemistry and Cell Motility 2:113-125 (1973).
- 30. Lunn, A.C., Lee, B-L. and Yannas, I.V., "Strain recovery of polyester and nylon 66 monofilaments under various temperature histories", Polymer Engineering and Science 14:610-615 (1974).
- 31. Gordon, P.L., Huang, C., Lord, R.C. and Yannas, I.V., "The far infrared spectrum of collagen", Macromolecules 7:954-956 (1974).
- 32. Yannas, I.V., "Nonlinear viscoelasticity of solid polymers (in uniaxial tensile loading)", Macromolecular Reviews 9:163-190 (1974).
- 33. Yannas, I.V., "A molecular mechanism for deformation in glassy and rubberlike polymers", Bulletin of the American Physical Society 20, Series II, No. 3, p. 402 (March 1975).
- 34. Yannas, I.V., Burke, J.F., Huang, C. and Gordon P.L., "Correlation of in vivo collagen degradation rate with in vitro measurements", Journal of Biomedical Materials Research 9:623-628 (1975).
- 35. Comminou, M. and Yannas, I.V., "Dependence of stress-strain nonlinearity of connective tissue on the geometry of collagen fibers", Journal of Biomechanics 9:427-433 (1976).
- 36. Huang, C. and Yannas, I.V., "Mechanical studies of enzymatic degradation of insoluble collagen fibers", Journal of Biomedical Materials Research, Symposium No. 8, 137-154 (1977).
- 37. Jansson, J-F. and Yannas, I.V., "The infrared dichroism of glassy polycarbonate at small strains", Journal of Polymer Science 15:2103-2111 (1977).
- 38. Silver, F.H., Yannas, I.V. and Salzman, E.W., "Glycosaminoglycan inhibition of collagen induced platelet aggregation", Thrombosis Research 13:267-277 (1978).
- 39. Bansil, R., Yannas, I.V. and Stanley, H.E., "Raman spectroscopy: a structural probe of glycosaminoglycans", Biochimica Biophysica Acta 541:535-542 (1978).
- 40. Markenscoff, X. and Yannas, I.V., "On the Stress-Strain Relation for Skin", Journal of Biomechanics 12:127-129 (1979).
- 41. Silver, F.H., Yannas, I.V. and Salzman, E.W., "In vitro blood compatibility of glycosaminoglycan-precipitated collagens", Journal of Biomedical Materials Research 13:701-716 (1979).
- 42. Yannas, I.V. and Burke, J.F., "Design of an Artificial Skin. I, Design Principles", Journal of Biomedical Materials Research 14, 65-68 (1980).
- 43. Yannas, I.V., Burke, J.F., Gordon, P.L., Huang, C. and Rubenstein, R.H., "Design of an Artificial Skin. II. Control of Chemical Composition", Journal of Biomedical Materials Research 14:107-131 (1980).
- 44. Dagalakis, N., Flink. J., Stasikelis, P., Burke, J.F. and Yannas, I.V., "Design of an Artificial Skin. Part III. Control of Pore Structure", Journal of Biomedical Materials Research 14:511-528 (1980).
- 45. Yannas, I.V., Burke, J.F., Warpehoski, M., Stasikelis, P., Skrabut, E.M., Orgill, D. and Giard, D.J., "Prompt, long-term functional replacement of skin", Transactions of American Society for Artificial Internal Organs 27:19-22 (1981).
- 46. Burke, J.F., Yannas, I.V., Quinby, W.C., Jr., Bondoc, C.C. and Jung, W.K., "Successful Use of a Physiologically Acceptable Artificial Skin in the Treatment of Extensive Burn Injury", Annals of Surgery 194:413-428 (1981).
- 47. Yannas, I.V., Burke, J.F., Orgill, D.P., Skrabut, E.M., "Wound Tissue Can Utilize a Polymeric Template to Synthesize a Functional Extension of Skin", Science 215:174-176 (1982).
- 48. Yannas, I.V. and Luise, R.R., "Distinction between Two Molecular Mechanisms of Deformation of Glassy Amorphous Polymers", Journal of Macromolecular Science-Physics B21:443-474 (1982).
- 49. Yannas, I.V., "What Criteria Should be Used for Designing Artificial Skin Replacements and How Well do the Current Grafting Materials Meet These Criteria?" J. Trauma 24:S29-S31 (1984).
- 50. Eman, W., Yannas, I.V. and Krueger, G.G., "Biology of Langerhans Cells: Further Insights into Origin and Migration", in Clinical Research 32:581A (1984).
- 51. Kardomateas, G. and Yannas, I.V., "A model for the Differing Crazing Behavior of Amorphous Polymer Glasses", Philosophical Magazine A52:39-50 (1985).
- 52. Murphy, G.F., Orgill, D.P., Hancock, W.W., Fonferko, E.B. and Yannas, I.V., "Morphological Reconstitution of Skin by Use of a Biodegradable Polymeric Graft", Laboratory Investigations 54:45A (1986).
- 53. Yannas, I.V., Lee, E., Orgill, D.P., Skrabut, E.M., and Murphy, G.F., "Synthesis and characterization of a model extracellular matrix that induces partial regeneration of adult mammalian skin", Proceedings of the National Academy of Sciences USA, 86:933-937 (l989).
- 54. Sylvester, M.F., Yannas, I.V., Salzman, E.W., and Forbes, M.J., "Collagen Banded Fibril Structure and the Collagen-Platelet Reaction", Thromb. Res. 55:135-148 (1989).
- 55. Murphy, G.F., Orgill, D.P., and Yannas, I.V., "Dermal Regeneration is Induced by Biodegradable Collagen-Glycosaminoglycan Grafts", Lab. Invest. 63:305-313 (1990).
- 56. Yannas, I.V., "Biologically Active Analogs of the Extracellular Matrix", Angewandte Chemie 29:20-35 (1990).
- 57. Ferdman, A.G. and Yannas, I.V., "The Scattering of Light from Histological Sections: A New Method for the Analysis of Connective Tissue." J. Investigative Dermatology, 100:710-716 (1993).
- 58. Yannas, I.V. (1994). "Applications of ECM analogs in surgery." J. Cell. Biochem. 56:188-191.
- 59. Louie, L.K., Yannas, I.V. and M. Spector (1994). "Development of a collagen-GAG copolymer implant for the study of tendon regeneration." Mat. Res. Soc. Symp. Proc. 331:19-24.
- 60. Shafritz, T.A., Rosenberg, L.C. and Yannas, I.V. (1994). "Specific effects of glycosaminoglycans in an analog of extracellular matrix that delays wound contraction and induces regeneration." Wound Rep. Reg., 2:270-276.
- 61. Yannas, I.V. (1995). "Tissue regeneration templates based on collagen-glycosaminoglycan copolymers." Adv. Polymer Sci. 122:219-244.
- 62. Chen, C.S., Yannas, I.V. and Spector, M. (1995). "Pore strain behavior of collagen-glycosaminoglycan analogues of extracellular matrix." Biomaterials, 16:777-783.
- 63. Faryniarz, D.A., Chaponnier, C., Gabbiani, G., Yannas, I.V. and M. Spector (1995). "Myofibroblasts in the healing lapine collateral ligament: Possible mechanisms of contraction." J. Ortho. Res., 14:228-237.
- 64. Yannas, I.V., Colt, J. and Wai, Y.C. (1996)."Wound contraction and scar synthesis during development of the amphibian Rana catesbeiana."Wound Rep. Reg., 4:31-41.
- 65. Ellis, D.L. and Yannas, I.V. (1996). Recent advances in tissue synthesis in vivo by use of collagen-glycosaminoglycan copolymers, Biomaterials, 17, No. 3, 291-299.
- 66. Chamberlain, L. J., I. V. Yannas, H.-P. Hsu and M. Spector (1997). Histological response to a fully degradable collagen device implanted in a gap in the rat sciatic nerve. Tissue Eng. 3:353-362.
- 67. Nehrer, S., Breinan, H.A., Ramappa, A., Shortkroff, S., Young, G., Minas, T., Sledge, C.B., Yannas, I.V. and Spector, M. (1997). Canine chondrocytes seeded in type I and type II collagen implants investigated In Vitro, J. Biomed Mater Res (Appl. Biomater.), 38:95-104.
- 68. Nehrer, S., Breinan, H.A., Ramappa, A., Young, G., Shortkkroff, S., Louie, L.K., Sledge, C.B., Yannas, I.V. and Spector, M. (1997). Matrix collagen type and pore size influence behavior of seeded canine chondrocytes, Biomaterials 18:769-776.
- 69. Spilker, M. H., I. V. Yannas, H.-P. Hsu, T. V. Norregaard, S. K. Kostyk and M. Spector (1997). The effects of collagen-based implants on early healing of the adult rat spinal cord. Tissue Eng. 3:309-317.
- 70. Yannas, I. V. (1998). Studies on the biological activity of the dermal regeneration template. Wound Rep. Reg. 6:518-524.
- 71. Brown, R. A., R. Prajapati, D. A. McGrouther, I. V. Yannas and M. Eastwood (1998). Tensional homeostasis in dermal fibroblasts: Mechanical responses to mechanical loading in three-dimensional substrates. J. Cell Physiol. 175:323-332.
- 72. Orgill, D. P. and Yannas, I. V. (1998). Design of an artificial skin. IV Use of island graft to isolate organ regeneration from scar synthesis and other processes leading to skin wound closure. J. Biomed. Mater. Res. 36:531-535.
- 73. Compton, C. C., C. E. Butler, I. V. Yannas, G. Warland and D. P. Orgill (1998). Organized skin structure is regenerated in vivo from collagen-GAG matrices seeded with autologous keratinocytes. J. Invest. Dermatol. 110:908-916.
- 74. Butler, C. E., D. P. Orgill, I. V. Yannas and C. C. Compton (1998). Effect of keratinocyte seeding of collagen-glycosaminoglycan membranes on the regeneration of skin in a porcine model. Plast. Reconstr. Surg. 101:1572-1579.
- 75. Chamberlain, L. J., I. V. Yannas, A. Arrizabalaga, H.-P. Hsu, T. V. Norregaard and M. Spector (1998). Early peripheral nerve healing in collagen and silicone tube implants: Myofibroblasts and the cellular response. Biomaterials 19:1393-1403.
- 76. Chamberlain, L. J., I. V. Yannas, H-P. Hsu, G. Strichartz and M. Spector (1998). Collagen-GAG substrate enhances the quality of nerve regeneration through collagen tubes up to level of autograft. Exp. Neurol. 154:315-329.
- 77. Orgill, D. P., C. Butler, J. F. Regan, M. S. Barlow, I. V. Yannas and C. C. Compton (1998). Vascularized collagen-glycosaminoglycan matrix provides a dermal substrate and improves take of cultured epithelial autografts. Plast. Reconstr. Surg. 102:423-429.
- 78. Brown, R. A., R. Prajapati, D. A. McGrouther, I. V. Yannas and M. Eastwood (1998). Tensional homeostasis in dermal fibroblasts: Mechanical responses to mechanical loading in three-dimensional substrates. J. Cell Physiol. 175:323-332.
- 79. Butler, C. E., I. V. Yannas, C. C. Compton and D. P. Orgill (1999). Comparison of cultured and uncultured keratinocytes seeded into a collagen-GAG matrix for skin replacement. Br. J. Plast. Surg. 52:127-132.
- 80. Chamberlain, L. J., I. V. Yannas, H.-P. Hsu and M. Spector (2000). Connective tissue response to tubular implants for peripheral nerve regeneration: The role of myofibroblasts. J. Comp. Neurol. 417:415-430.
- 81. Chamberlain, L. J., I. V. Yannas, H-P. Hsu, G. R. Strichartz and M. Spector (2000). Near-terminus axonal structure and function following rat sciatic nerve regeneration through a collagen-GAG matrix in a 10-mm gap. J.Neurosci. Res.60:666-677.
- 82. Hsu, W-C., Spilker M. H., Yannas I. V., and Rubin P. A. D. (2000). Inhibition of conjunctival scarring and contraction by a porous collagen-GAG implant. Invest. Ophthalmol. Vis. Sci. 41:2404-2411.
- 83. Torres, D. S., T. M. Freyman, I. V. Yannas and M. Spector (2000). Tendon cell contraction of collagen-GAG matrices in vitro: effect of cross-linking. Biomaterials 21:1607-1619.
- 84. Yannas, I. V. (2000). Synthesis of organs: In vitro or in vivo? Proc. Natl. Acad. Sci. USA 97:9354-9356.
- 85. Freyman, T. M., I. V. Yannas and L. J. Gibson (2001). Cellular materials as porous scaffolds for tissue engineering. Progr. Mat. Sci. 46:273-282.
- 86. Spilker MH, Asano K, Yannas IV, Spector M. (2001). Contraction of collagen-glycosaminoglycan matrices by peripheral nerve cells in vitro. Biomaterials. 22:1085-93
- 87. Freyman, T. M., I. V. Yannas, Y-S. Pek, R. Yokoo and L. J. Gibson (2001). Micromechanics of fibroblast contraction of a collagen-GAG matrix. Exp Cell Res. 269:140-53.
- 88. Zaleskas, J. M., B. Kinner, T. M. Freyman, I. V. Yannas, L. J. Gibson and M. Spector (2001). Growth factor regulation of smooth muscle actin expression and contraction of human articular chondrocytes and meniscal cells in a collagen-GAG matrix. Exp Cell Res. 270:21-31.
- 89. Spilker MH, Yannas IV, Kostyk SK, Norregaard TV, Hsu H-P and Spector M (2001). The effect of tubulation on healing and scar formation after transection of the adult rat spinal cord. Restor. Neurol. Neurosci., 18:23-38.
- 90. Freyman TM, Yannas IV, Yokoo R, Gibson LJ. (2001). Fibroblast contraction of a collagen-GAG matrix. Biomaterials. 22:2883-91.
- 91. Freyman, T. M., Yannas, I. V., Yokoo R., and Gibson L. J. (2002). Fibroblast contractile force is independent of the stiffness which resists the contraction. Exp. Cell Res. 272:153-162.
- 92. Samuel, R. E., C. R. Lee, S. Ghivizanni, C. H. Evans, I. V. Yannas, B. R. Olsen and M. Spector (2002). Delivery of plasmid DNA to articular chondrocytes via novel collagen-GAG matrices. Human Gene Therapy. 13:791-802.
- 93. Sethi, K. K., I. V. Yannas, V. Mudera, M. Eastwood, C. McFarland and R. A. Brown (2002). Evidence for sequential utilization of fibronectin, vitronectin, and collagen during fibroblast-mediated collagen contraction. Wound Rep. Reg. 10:397-408.
- 94. Zaleskas, J. M., B. Kinner, T. M. Freyman, I. V. Yannas, L. J. Gibson and M. Spector (2003). Contractile forces generated by articular chondrocytes in collagen-glycosaminoglycan matrices. Biomaterials. 2004 Mar;25(7-8):1299-308.
- 95. YS Pek, M Spector, IV Yannas and LJ Gibson. 2003. Degradation of a Collagen Chondroitin-6-Sulfate Matrix by Collagenase and by Chondroitinase. Biomaterials. 2004 Feb;25(3):473-82.
- 96. Yannas, I. V. and Hill, BJ (2004). Selection of biomaterials for peripheral nerve regeneration using data from the tubulation model. Biomaterials. 25:1593-600.
- 97. O'Brien, F. J., B. A. Harley, I. V. Yannas, and L. Gibson. 2004. Influence of freezing rate on pore structure in freeze-dried collagen-GAG scaffolds. Biomaterials 25:1077-1086.
- 98. Harley BA, Spilker MH, Wu JW, Asano K, Hsu HP, Spector M, Yannas IV. (2004). Optimal degradation rate for collagen chambers used for regeneration of peripheral nerves over long gaps. Cells Tissues Organs. 176:153-65.
- 99. I.V. Yannas. Synthesis of Tissues and Organs. ChemBioChem. 2004. 4:10-23.
- 100. Vickers SM. Johnson LL, Zou LQ, Yannas IV, Gibson LJ and Spector M. (2004). Expression of a-smooth muscle actin by and contraction of cells derived from synovium. Tissue Eng. 10: 1214-1223.
- 101. Lynn AK, Yannas IV, Bonfield W. (2004). Antigenicity and immunogenicity of collagen. J Biomed Mater Res. 71B(2):343-54.
- 102. Veilleux NH, Yannas IV, Spector M. (2004). Effect of passage number and collagen type on the proliferative, biosynthetic, and contractile activity of adult canine articular chondrocytes in type I and II collagen-glycosaminoglycan matrices in vitro. Tissue Eng. 10:119-27.
- 103. Zhang M and IV Yannas (2005) Peripheral nerve regeneration. Adv. Biochem. Engin./Biotechnol. 94:67-89.
- 104. O'Brien FJ, Harley BA, Yannas IV, Gibson LJ. (2005). The effect of pore size on cell adhesion in collagen-GAG scaffolds. Biomaterials. 26(4):433-41.
- 105. Yannas IV (2005). Facts and theories of organ regeneration. Adv. Biochem. Engin./Biotechnol. 93:1 -31.
- 106. Chen P, Marsilio E, Goldstein RH, Yannas IV, and Spector M.(2005). Formation of Lung Alveolar-Like Structures in Collagen-Glycosaminoglycan Scaffolds in Vitro. Tissue Eng. 11:1436-1448.
- 107. Yannas IV (2005). Similarities and differences between induced organ regeneration in adults and early foetal regeneration. J Roy Soc Interface 2:403-417.
- 108. Harley BA, Hastings AZ, Yannas IV, Sannino A. (2006). Fabricating tubular scaffolds with a radial pore size gradient by a spinning technique. Biomaterials. 27(6):866-74.
- 109. Farrell, A, O’Brien F.J., Doyle P, Fischer J, Yannas I, Harley BA, O’Connell B, Prendergast PJ, Campbell VA. A collagen-glycosaminoglycan scaffold supports adult rat mesenchymal cell differentiation along osteogenic and chondrogenic routes. (2006). Tissue Eng. 12:461-468.
- 110. Yannas IV (2006). Artificial skin and dermal equivalents. In Tissue Engineering and Artificial Organs, The Biomedical Engineering Handbook, 3rd ed., JD Bronzino, Ed. Chap. 75, pp. 75-1 to 75-15. Boca Raton: Taylor Francis.
- 111. Yannas IV (2006). Biologically active scaffolds based on collagen-GAG copolymers. In Scaffolds in Tissue Engineering, PX Ma and J Elisseeff, Eds. Chap. 1, pp. 3-12. Boca Raton: Taylor and Francis.
- 112. Soller EC, Yannas IV (2006). Induced regeneration of skin and peripheral nerves. In The Diabetic Foot, 2nd ed., Veves A, et al., editors, Ch. 5, pp. 83-103, Totowa, NJ: Humana Press.
- 113. Ingber DE, Mow VC, Butler D, Niklason L, Huard J, Mao J, Yannas I, Kaplan D, Vunjak-Novakovich G. Tissue engineering and developmental biology: going biomimetic. (2006). Tissue Engineering 12:3265-3283.
- 114. O'Brien FJ, Harley BA, Waller MA, Yannas IV, Gibson LJ, Prendergast PJ. (2007) The effect of pore size on permeability and cell attachment in collagen scaffolds for tissue engineering. Technol Health Care.15(1):3-17.
- 115. Yannas IV, Kwan MD, Longaker MT. (2007) Early fetal healing as a model for adult organ regeneration. Tissue Eng. 13(8):1789-98; also Jan 1; [Epub ahead of print];
- 116. Yannas IV, M Zhang and MH Spilker (2007). Standardized criterion to analyze and directly compare various materials and models for peripheral nerve regeneration. J Biomater Sci Polymer Edn 18:943-966.
- 117. Madaghiele M, Sannino A, Yannas IV, Spector M. Collagen-based matrices with axially oriented pores. J Biomed Mater Res A. 2007 Sep 26;
- 118. Yannas IV. (2008) Organ Regeneration. In McGraw-Hill Yearbook of Science and Technology. pp. 245-7.
- 119. Harley BA, Kim HD, Zaman MH, Yannas IV, Lauffenburger DA, Gibson LJ. Micro architecture of three dimensional scaffolds influences cell migration behavior via junction interactions. Biophys J.(2008) Oct;95(8):4013-24.
- 120. Yannas IV (2008). Tissues and Organs, Synthesis of. Wiley Encyclopedia of Chemical Biology.
- 121. Harley BA, Lynn AK, Wissner-Gross Z, Bonfield W, Yannas IV, Gibson LJ. Design of a Multiphase Osteochondral Scaffold. III: Fabrication of layered scaffolds with soft interfaces. J Biomed Mater Res A, (2010) Mar 1;92(3): 1078-93.
- 122. Harley BA, Lynn AK, Wissner-Gross Z, Bonfield W, Yannas IV, Gibson LJ. Design of a Multiphase Osteochondral Scaffold. II: Fabrication of a mineralized collagen-GAG scaffold. J Biomed Mater Res A. (2010) Mar 1;92(3):1066-67.
- 123. Yannas IV (2009). Principles of skin regeneration. Chapter in Treatments of Skin Loss, Woodhead, London, UK.
- 124. Lynn AK, Best SM, Cameron RE, Harley BA, Yannas IV, Gibson LJ, Bonfield W. Design of a multiphase osteochondral scaffold. I. Control of chemical composition. J Biomed Mater Res A. (2010) Mar 1;92(3):1057- 65.
- 125. Song G, Nguyen DT, Pietramaggiori G, Scherer S, Chen B, Zhan Q, Ogawa R, Yannas IV, Wagers AJ. Murphy GF, Kimberly R, Schanche RA, Orgill DP, Murphy GF (2009). Hematopoietic Participation in Early Cutaneous Wound Healing Evaluated in a Parabiotic Model. Accepted for publication in Wound Repair and Regeneration.
- 126. Lynn AK, Best SM, Cameron RE, Harley BA, Yannas IV, Gibson LJ, Bonfield W. (2009) Design of a Multiphase Osteochondral Scaffold I: Control of Chemical Composition. J Biomed Mater Res A. 2010 Mar 1;92(3):1057-65.
- 127. Sannino A, Silvestri L, Madaghiele M, Harley B, Yannas IV. (2009). Modeling the fabrication process of micropatterned macromolecular scaffolds for peripheral nerve regeneration. J Applied Polymer Science. Accepted for publication.
- 128. I.V. Yannas, D.S. Tzeranis, B.A. Harley and P.T.C. So, (2010). Biologically active collagen-based scaffolds: Advances in processing and characterization. Philosophical Transactions of Royal Society. A 368 2123-2139, 2010.
- 129. Tzeranis DS, Roy A, So PT, Yannas IV. An optical method to quantify the density of ligands for cell adhesion receptors in three-dimensional matrices. J R Soc Interface. 2010 Jul 29. [Epub ahead of print]
- 130. Yannas IV, Orgill DP, Burke JF. Template for skin regeneration. Plast Reconstr Surg. 2011 Jan;127 Suppl 1:60S-70S. PMID: 21200274
- 131. Eric C. Soller, Dimitrios S. Tzeranis, Kathy Miu, Peter T.C. So, Ioannis V. Yannas. Biomaterials 33 (2012) 4783-4791. Common features of optimal collagen scaffolds that disrupt wound contraction and enhance regeneration both in peripheral nerves and in skin.
- 132. Yannas IV. Emerging rules for inducing organ regeneration. Biomaterials. 34:321-606 (2013).
- 133. I.V. Yannas, D. Tzeranis and P.T. So. Surface Biology Of Collagen Scaffold Explains Blocking Of Wound Contraction And Regeneration Of Skin And Peripheral Nerves. Biomedical Materials, 11:014106 (2015).
- 134. Tzeranis DS, Soller EC, Buydash MC, So PT, Yannas IV. In situ quantification of surface chemistry in porous collagen biomaterials. Ann. Biomed. Eng. 44:803-15 (2016).
- 135. Yannas IV, Tzeranis DS, So PTC. Regeneration of injured skin and peripheral nerves requires control of wound conraction, not scar formation. Wound Repair Regen. 25:177-191 (2017). doi: 10.1111/wrr.12516. Epub 2017 Apr 27
- 136. Yannas IV, Tzeranis DS, So PTC. (2018) Regeneration mechanism for skin and peripheral
nerves clarified at the organ and molecular scales. Current Opinion in Biomedical Engineering,
6:1-7.
-
137.
Yannas IV. (2018). Hesitant steps from the artificial skin to organ regeneration. Regenerative Biomaterials. 5(4):189-195. doi: 10.1093/rb/rby012. Epub 2018 Jun 26. -
138. Kourgiantaki A, Tzeranis DS, Karal K, Psilodimitrakopoulo S, Niko M, Yannas IV, Stratakis E, Sidiropoulou K, Charalampopoulos I, and Gravanis A (2020). Neural stem cell delivery via porous collagen scaffolds promotes neuronal differentiation and locomotion recovery in spinal cord injury. npj Regenerative Medicine. 5, 1-14.
C. Proceedings of Refereed Conferences:
- 1. Yannas, I.V. and Gonzalez, R.N., "Phenomenological Characterization of a Paper-Coating Suspension", Transactions of the Society of Rheology 6:143-155 (1962).
- 2. Yannas, I.V., "The Glass Transformation of a Protein", First Northeast Regional Meeting of the Am. Chem. Soc., Abst. No. 255, p. 116 (October 13-15, 1968).
- 3. Yannas, I.V., Lunn, A.C., and Sung, N-H., "Mechanical Softening: A Precursor to Cold Drawing", Polymer Preprints 10:1113-1116 (1969).
- 4. Yannas, I.V., Arghyros, S., Sheik, A. and Turai, T., "Crosslinking of a Soluble Collagen by Dehydration", Abstracts, 3rd Internat'l Biophysics Congress, Cambridge, MA (Aug. 29-Sep. 3, 1969).
- 5. Yannas, I.V. and Arends, C.B., "Massive internal fracture of amorphous polyester", Abstracts of Scientific Communications presented at the 4th IUPAC Microsymposium on Macromolecules, Prague, Abstract G4, (September 1-4, 1969).
- 6. Yannas, I.V., "A critical modulus criterion for the onset of yielding in polymers", Abstracts of Scientific Communications presented at the 4th IUPAC Microsymposium on Macromolecules, Prague, Abstract J1, (September 1-4, 1969).
- 7. Yannas, I.V., Lunn, A.C., and Sung, N-H., "Mechanical Softening: a precursor to cold drawing", Abstracts of Scientific Communications presented at the 4th IUPAC Microsymposium on Macromolecules, Prague, Abstract J2, (September 1-4, 1969).
- 8. Yannas, I.V., Arghyros, S. and Huang, C., "Viscoelastic behavior of materials based on collagen and gelatin", Abstracts of Fifth Annual Meeting of the Association for the Advancement of Medical Instrumentation, Boston (March 23-25, 1970).
- 9. Yannas, I.V., Huang, C. and Olson, D.A., "Materials science of collagen, gelatin and protein composites", Proceedings of the 23rd Annual Conference on Engineering in Medicine and Biology Washington, p. 157 (November 15-19, 1970).
- 10. Yannas, I.V. and Lunn A.C., "Rupture and formation of secondary valence bonds during deformation of polycarbonate", Polymer Preprints 12:584-588 (1971).
- 11. Yannas, I.V. and Huang, C., "Fracture of Tendon Collagen", Polymer Preprints A2:589-591 (1971).
- 12. Yannas, I.V., Lunn, A.C. and Doyle, M.J., "The molecular basis of the transition from linear to nonlinear viscoelatic behavior in glassy polycarbonate", Polymer Preprints 13:96-100 (1972).
- 13. Yannas, I.V., Lunn, A.C. and Doyle, M.J., "The molecular basis of the transition from linear to nonlinear viscoelastic behavior of polymers", Abstracts of IUPAC Symposium on Macromolecules, Helsinki, Abstract, p. 355 (July 2-7, 1972).
- 14. Yannas, I.V. and Olson, D.A., "A linear model for mechanochemical transduction in human physiology", Proceedings of the 3rd Annual Meeting of the Biomedical Engineering Society, Baltimore, Abstract 9, (April 7-8, 1972).
- 15. Yannas, I.V., Sung, N-H, Olson, D.A. and Huang, C., "Physicochemical definition of collagen as a polymeric substance in the solid state", Abstracts of IUPAC Symposium on Macromolecules, Helsinki, Abstract, p. 357 (July 2-7, l972).
- 16. Yannas, I.V., "Characterization techniques for the molecular design of polymeric implants", Proceedings of Biomedical Materials Conference, Brighton, Utah, p. 133 (November 9-19, 1972).
- 17. Yannas, I.V. and Sung, N-H., "Optical rotatory dispersion of collagen in the solid state: A sensitive assay of helical content", Polymer Preprints 13:123-127 (1972).
- 18. Yannas, I.V., "Nonlinear viscoelasticity of polymers", Polymer Preprints 14:803-812 (1973).
- 19. Yannas, I.V., "Molecular interpretation of deformation in glassy polymers", Proceedings of IUPAC International Symposium on Macromolecules, Rio de Janeiro, 265-285 (July 26-31, 1974).
- 20. Yannas, I.V., Burke, J.F., Huang, C., and Gordon, P.L. "Suppression of in vivo degradability and of immunogenicity of collagen by reaction with glycosaminoglycans", Polymer Preprints 16(2):209-214 (1975).
- 21. Yannas, I.V. and Lunn, A.C., "Inter- versus intramolecular energy barriers during deformation of glassy amorphous polymers", Polymer Preprints 16(2):564-569 (1975).
- 22. Yannas, I.V. and Silver, F., "Thromboresistant analogs of vascular tissue" Polymer Preprints 16(2):529-534 (1975).
- 23. Huang, C. and Yannas, I.V., "Enzymatic stress relaxation of collagen and gelatin fibers", Coatings and Plastics Reprints (ACS) 36(2):243-248 (1976).
- 24. Yannas, I.V., Burke, J.F., Huang, C. and Gordon, P.L.,"Control of biodegradation rate in design of collagenous skin substitutes", Abstracts of Third Annual Meeting of the Society for Biomaterials, New Orleans, Abstract 107 (April 15-19, 1977).
- 25. Yannas, I.V., Silver, F.H. and Salzman, E.W., "Inhibition of the collagen-platelet reaction following collagen lattice disorganization by glycosaminoglycans", Transactions of 4th Annual Meeting of the Society for Biomaterials, San Antonio, p. 68 (April 29-May 2, 1978).
- 26. Yannas, I.V., Burke, J.F., Umbreit, M. and Stasikelis, P., "Progress in Design of an Artificial Skin", Fed. Proc. 38:988 (1979).
- 27. Yannas, I.V. and Burke, J.F., "Artificial Skin Design: Performance of Stage 1", Abst., First World Biomaterials Congress, Baden near Vienna, Austria, p. 1.4.1 (1980).
- 28. Yannas, I.V. and Burke, J.F., "Wound Closure by a Single-Application Skin Substitute", Abst., AIChE 73rd Annual Meeting, Chicago, IL, (1980), p. T-45.
- 29. Yannas, I.V., Burke, J.F., Trelstad, R.L., Stasikelis, P., Warpehoski, M., Skrabut, E., Giard, D., "Permanent Closure of Deep Wound with a Polymeric Membrane", 27th International Symposium on Macromolecules, Strasbourg, July 6-9, 1981.
- 30. Yannas, I.V. and Burke, J.F., Use of Stage-1 Artificial Skin in early, complete closure of full-thickness wounds, Abstracts, Amer. Soc. Artif. Int. Org. 10:75 (1981).
- 31. Yannas, I.V., Burke, J.F., and Skrabut, E.M., "Artificial Skin: Early, Single-Application Closure of Full-thickness Skin Wounds Without Use of Autograft", Fed. Proc., Am. Soc. Exp. Biol. 40:520 (1981).
- 32. Yannas, I.V. and Burke, J.F., "Performance of Stage 1 Artificial Skin with Human Subjects: Preliminary Results", Transactions of the Seventh Annual Meeting of the Society for Biomaterials, 4:91 (1981).
- 33. Yannas, I.V., Burke, J.F., Orgill, D.P. and Skrabut, E.M., "Regeneration of Skin Following Closure of Deep Wounds with a Biodegradable Template", Trans. Soc. Biomaterials 5:24-29 (1982).
- 34. Yannas, I.V., Burke, J.F., Chen, E., Orgill, D.P., and Skrabut, E.M., "Stage 2 Artificial Skin: A Polymeric Template for Regeneration of New Skin", IUPAC Proceedings, 28th Macromolecular Symposium, Amherst, MA July 12-16, 1982.
- 35. Yannas, I.V. Salzman, E.W., Forbes, M.J., Sylverster, M.F., "Thrombogenic/Nonthrombogenic Collagen Fibers Effect of Pretreatment pH", Soc. of Biomater. Abs. (1982).
- 36. Yannas, I.V., Salzman, E.W., Sylvester, M.L. and Forbes, M.J., "The Effect of Quaternary Structure on the Collagen-Platelet Reaction", American Chemical Society Meeting, Seattle, Washington (March 20-25, 1983).
- 37. Yannas, I.V., Burke, J.F., Orgill, D., and Skrabut, E.M., "Skin Regeneration by Use of a Bioreplaceable Polymeric Template", Trans., 2nd World Congress on Biomats. VII, p. 35 (1984).
- 38. Yannas, I.V., Orgill, D.P., Silver, J., Norregaard, T.V., Zervas, N.T. and Schoene, W.C., "Polymeric Template Facilitates Regeneration of Sciatic Nerve Across 15-mm Gap", Transactions of the Society of Biomaterials 8:1946 (1985); Proceedings, Am. Chem. Soc. Div. Polymeric Mat. 53:216-218 (1985).
- 39. Yannas, I.V. and Orgill, D.P., "A Fifth Route to Organ Repair", Proceedings, Society of Plastics Engineers Congress, 2233-2238 (1985).
- 40. Yannas, I.V., Orgill, D.P., Silver, J., Norregaard, T.V., Zervas, N.T. and Schoene, W.C., "Morphometric Evidence of Peripheral Nerve Regeneration Following Grafting with a Polymeric Template", Transactions Society Biomaterials 9:174 (1986).
- 41. Yannas, I.V., Krarup, C., Chang, A., Norregaard, T.V., Zervas, N.T., Sethi, R., "Conduction Properties of Nerve Fibers Regenerated Across Gap Bridged by Biodegradable Polymer Matrix", Soc. of Neuroscience Abstract 13:1043 (1987).
- 42. Yannas, I.V., Norregaard, T.V., Silver, J., Zervas, N.T., Kirk, J.F. and Colt, J.J., "Relations Between Properties of Collagen-Glycosaminoglycan Graft and Morphology of Regenerating Nerve. Part I." Trans. Soc. Biomaterials 10:6 (l987).
- 43. Ferdman, A. and Yannas, I.V., "Small Angle Light Scattering From Histological Sections of Connective Tissue", Trans. Soc. Biomaterials 10:207 (l987).
- 44. Yannas, I.V., "A Mechanism of Tissue Regeneration Using a Biodegradable Template, Part I." Trans. Soc. Biomaterials 10:l75 (l987).
- 45. Yannas, I.V., Lee, E., Orgill, D.P., Ferdman, A., Skrabut, E.M. and Murphy, G.F., "De Novo Synthesis of Skin", Proc. Am. Chem. Soc. Div. Polym. Mat. 57:28-32 (l987).
- 46. Yannas, I.V., Lee, E., E.M. Skrabut, E.M., Orgill, D.P., and G.F. Murphy, G.F., "Characterization of a Polymeric Matrix for Regeneration of Skin", abstract, Journal of Cell Biology 105:223a, (1987).
- 47. Yannas, I.V., "Polymeric Matrices Induce Regeneration of Mammalian Skin: Abs. Int. Symp. Cutaneous Dev., Aging Repair, Padova, p. 29 (1987).
- 48. Chang, A.S., Yannas, I.V., Krarup, C., Sethi, R., Norregaard, T.V., Zervas, N.T., "Conduction Properties in Peripheral Nerve Fibers Regenerated by Biodegradable Polymer Matrix", MRS Symp. Abs. p. 34l (1987).
- 49. Yannas, I.V., Lee, E., Orgill, D.P., Skrabut, E.M., and Ferdman, A., "Certain Critical Structural Parameters of Polymeric Templates for Regeneration of Skin", MRS Symp. Abs. p. 341 (1987).
- 50. Ferdman, A.G and Yannas, I.V., "Collagen Fiber Orientation in Tissue as Measured by Small-Angle Light Scattering", MRS Symp. Abs. p. 356 (1987).
- 51. Yannas, I.V., "Properties of a Polymeric Matrix for the Regeneration of Skin and Nerve", Transactions, 3rd World Biomaterials Congress, XI p. 40 (1988).
- 52. Chang, A.S., Yannas, I.V., Krarup, C., Sethi, R., Norregaard, T.V., and Zervas, N.T., "Polymeric Templates for Peripheral Nerve Regeneration. Electrophysiological Study of Functional Recovery.", Proceedings of the ACS Division of Polymeric Materials: Science and Engineering, 59:906-910 (1988).
- 53. Yannas, I.V., Chang, A.S., Krarup, C., Sethi, R., Norregaard, T.V. and Zervas, N.T., "Conduction Properties of Peripheral Nerves Regenerated by Use of Copolymer Matrices with Different Biodegradation Rates", Soc. of Neurosci. Abs. 14:165 (1988).
- 54. Carman, L.S., Schneider, G.E., and Yannas, I.V., "Extension of critical age for retinal axon regeneration by polymer bridges conditioned in neonatal cortex", Soc. of Neurosci. Abs. 14:498 (1988).
- 55. Yannas, I.V., Chang, A.S., Krarup, C., Norregaard, T.V. and Zervas, N.T., "Environmental Preference of Regenerating Axons. Effects of Matrix Pore Orientation, Pore Diameter and Degradation Rate", Am. Soc. for Cell Biology Abs. 107:732a (1989).
- 56. Loree, H.M., Yannas, I.V., Mikic, B., Chang, A.S., Perutz, S.M., Norregaard, T.V., and Krarup, C., "A Freeze-Drying Process for Fabrication of Polymeric Bridges for Peripheral Nerve Regeneration", Proc. 15th Annual Northeast Bioeng. Conf., pp. 53-54 (1989).
- 57. Chang, A.S., Yannas, I.V., Krarup, C., Sethi, R., Norregaard, T.V., and Zervas, N., "Conduction Properties in Peripheral Nerve Fibers Regenerated by Biodegradable Polymer Matrix", Mat. Res. Soc. Symp. 110:3-8 (1989).
- 58. Yannas, I.V., Chang, A., Loree, H., Perutz, S., Krarup, C., and Norregaard, T.V., "Regeneration of Peripheral Nerves in Controlled Polymeric Environments", Trans. Soc. Biomat. 12:119 (1989).
- 59. Yannas, I.V., Chang, A.S., Perutz, S., Krarup, C., Norregaard, T.V., and Zervas, N.T., "Requirement for a 1-µm Pore Channel Opening During Peripheral Nerve Regeneration Through a Biodegradable Chemical Analog of ECM", Soc. for Neurosci. Abs. 15:1257 (1989).
- 60. Yannas, I.V., Chang, A.S., Perutz, S., Krarup, C., Norregaard, T.V., and Zervas, N.T., "Requirement for a 1-µm Pore Channel Opening During Peripheral Nerve Regeneration Through a Biodegradable Chemical Analog of ECM. Proc. Am. Soc. Div. Polym. Mat. 62:583-586 (1990).
- 61. Yannas, I.V., Orgill, D.P., and Murphy, G.F., "Chemical Analogs of the Extracellular Matrix. Cell-matrix Interactions." Proc. Am. Soc. Div. Polym. Mat. 62:801-803 (1990).
- 62. Loree, H.M., Yannas, I.V., Mikic, B., Change A.S., and Perutz, S.M., "Peripheral Nerve Regeneration Using ECM Analogs. Control of Orientation Pore Channels." AIChE l99l Annual Meeting Extended Abstract, p. 3 (November l7-22, 1991).
- 63. Yannas, I.V., Skrabut, E.M., Orgill, D.P., and Murphy, G.F., "De Novo Synthesis of Skin. Part II. Island Grafts." AIChE l99l Annual Meeting Extended Abstract, p. 4 (November l7-22, 1991).
- 64. Yannas, I.V., Skrabut, E.M. and Orgill, D.P., "In Vivo Synthesis of Organs by Use of ECM Analogs", MRS Symp Abs. p. 563 (1991).
- 65. Troxel, K.S. and Yannas, I.V., "Myofibroblast axis orientation (alignment) is required for wound contraction." J. Cell Biology Abstracts 115 (3, Pt. 2):114a (1991).
- 66. Yannas, I.V., "Templates for organ regeneration" Biomaterials & Intelligent Materials. Technological Aspects & Medical Applications. Syllabus & Final Program. p. 109 (September 21-26, 1992).
- 67. Yannas, I.V., "Templates for organ regeneration", Trans. and Final Program, Fourth World Biomaterials Congress p. IL5 (April 24-28, 1992).
- 68. Louie, L.K., Yannas, I.V., and Spector, M., “Development of a Collagen-GAG Copolymer Implant for the Study of Tendon Regeneration”, Mat. Res. Soc. Symp. Proc. 331:19-24 (1994).
- 69. Butler, C.E., Orgill, D.P., Compton, C. and Yannas, I.V. (1996). Effects of cell culturing on keratinocyte-seeded collagen-glycosaminoglycan matrix skin replacement in full-thickness porcine wounds, Surg. For. 47: 752-754.
- Several abstracts presented to Society for Biomaterials and other societies during the period 1996-2016.
D. Other Major Publications (mostly chapters in books during 1971-2004; after 2004, chapters in books are listed under “Publications” above):
- 1. Tobolsky, A.V. and Yannas, I.V., "Thermodynamics of polymer solutions", Chapter 4 in Polymer Science and Materials, Vol. 1, 67-82 (1971).
- 2. Yannas, I.V., "Physical chemistry of collagen in the solid state", Chapter 3 in Biomedical Physics and Biomaterials Science, pp. 41-63 (1972).
- 3. Yannas, I.V., "Use of Artificial Skin in Wound Management", Chapter 15 in The Surgical Wound, P. Dineen, ed., Lea and Febiger, Philadelphia, pp. 171-190 (1981).
- 4. Yannas, I.V., Burke, J.F., Trelstad, R.L., Stasikelis, P., Warpehoski, M., Skrabut, E. and Giard, D., "Single-Application Closure of Deep Skin Wounds with a Polymeric Membrane", in 1981 Advances in Bioengineering, D.C. Viano, editor, pp. 207-209 (1981).
- 5. Yannas, I.V. and Burke, J.F., "Artificial Skin Design: Permanent Closure of Full Thickness Skin Wounds", in Biomaterials 1980, G.D. Winter, D.F. Gibbons and H. Plenck, Jr., John Wiley, New York, pp. 635-640 (1982).
- 6. Yannas, I.V. and Luise, R.R., "The Strophon Theory of Deformation of Glassy Amorphous Polymers. Application to Small Deformations", in The Strength and Stiffness of Polymers, A.E. Zachariades and R.S. Porter, editors, Dekker, New York, Chapter 6, pp. 255-292 (1982).
- 7. Yannas, I.V., Burke, J.F., Wapehoski, M., Stasikelis, P., Skrabut, E.M., Orgill, D.P., Giard, D., "Design Principles and Preliminary Clinical Performance of an Artificial Skin", in Biomaterials: Interfacial Phenomena and Applications, N. Peppas and S.L. Cooper, editor, Advances in Chemistry Series, ACS, Washington, No. 199, 475-481 (1982).
- 8. Yannas, I.V., Orgill, D.P., Skrabut, E.M. and Burke, J.F., "Skin Regeneration with a Bioreplaceable Polymeric Template", Chapter 13 in Polymeric Materials and Artificial Organs, C.G. Gebelein, editor, ACS, Washington, pp. 191-197 (1984).
- 9. Yannas, I.V. and Orgill, D.P., Artificial Skin: A Fifth Route to Organ Repair, in Polymeric Biomaterials, E. Piskin and A.S. Hoffmann, eds., Martinas, Nijhogg, Dordrecht, pp. 221-230 (1986).
- 10. Yannas, I.V., "Skin", in McGraw-Hill Yearbook of Science and Technology, S.P. Parker, editor, McGraw-Hill, NY pp. 422-423 (l987).
- 11. Yannas, I.V., Orgill, D.P., Silver, J., Norregaard, T.V., Zervas, N.T., and Schoene, W.C., "Regeneration of Sciatic Nerve Across 15-mm Gap by Use of a Polymeric Template", in Advances in Biomedical Polymers, Charles G. Gebelein, editor, Plenum Publishing Corp., NY, pp. 1-9 (l987).
- 12. Yannas, I.V., "Regeneration of Skin and Nerves by use of Collagen Templates", in Vol. III, Collagen: Biotechnology, M. Nimni, editor, CRC Press, Boca Raton, FL, Chapt. 4, pp. 87-115 (l988).
- 13. Yannas, I.V., "Control of Kinetics and Mechanism of Skin Wound Healing By Use of a Bilayer Polymeric Membrane", in Engineering Applications of New Composites, S.A. Paipetis and G.C. Papanicolaou, eds., Omega Scientific, Wallingford, pp. 14-16 (l988).
- 14. Yannas, I.V., Lee, E., and Dionne Bentz, M., "Control of Skin Wound Contraction Rate by Critically Insoluble Collagen Matrices", in Applied Bioactive Polymeric Materials, C.G. Gebelein, C.E. Carraher, and V.R. Foster, eds., Plenum Press, NY, pp. 313-318 (1988).
- 15. Yannas, I.V., "Skin, Regeneration Templates" in Encyclopedia of Polymer Science and Engineering, Vol. 15, second edition, Wiley, NY, pp. 317-334, (1989).
- 16. Yannas, I.V., "Certain Biological Implications of Mammalian Skin Regeneration by a Model Extracellular Matrix", in Cutaneous Development, Aging and Repair, Davidson, J.M. and Abatangelo, G., editors, Liviana Press, Padova, pp.131-139 (1989).
- 17. Chang, A.S., Yannas, I.V., Perutz, S., Loree, H., Sethi, R.R., Krarup, C., Norregaard, T.V., Zervas, N.T., and Silver, J., "Electrophysiological Study of Recovery of Peripheral Nerves Regenerated by a Collagen-Glycosaminoglycan Copolymer Matrix", in Progress in Biomedical Polymers, C.G. Gebelein, ed., Plenum Press, NY, pp. 107-120, (1990).
- 18. Yannas, I.V., "Biologically Active Analogs of the Extracellular Matrix", Chapter 5 in Materials Science and Technology, D.F. Williams, editor, pp. 179-208 (1991).
- 19. Yannas, I.V., Chang, A.S., Perutz, S., Krarup, C., Norregaard, T.V., and Zervas, N.T., "Requirement for a 1-µm Pore Channel Opening During Peripheral Nerve Regeneration Through a Biodegradable Chemical Analog of ECM." in Biotechnology and Polymers, C.G. Gebelein, ed., Plenum Press, NY, pp. 275-279 (1991).
- 20. Chang, A.S. and Yannas, I.V., "Peripheral Nerve Regeneration", in Neuroscience Year (Supplement 2 to the Encyclopedia of Neuroscience), B. Smith and G. Adelman, eds., Birkhaüser Boston, pp. 125-126 (1992).
- 21. Luise, R. and Yannas, I.V., "Mechanics of Strophons in Glassy Amorphous Polymers. Unified view of Stiffness, Yielding and Crazing Behavior.", in Computational Modeling of Polymers, J. Bicerano, ed., Marcel Dekker, Inc., New York, NY pp. 191-218 (1992).
- 22. Yannas, I.V. "Tissue Regeneration by Use of Collagen-Glycosaminoglycan Copolymers", in Clinical Materials, J.A. Werkmeister and J.A.M. Ramshaw, eds., Elsevier Science Publishers, 9:179-187 (1992).
- 23. Yannas, I. V. (1996). Natural Materials, Chapter 2.7 in Biomaterials Science: An Introduction to Materials in Medicine, B. Ratner, A. Hoffman, J. Lemons and F. Schoen, eds., Academic Press, NY, pp. 84-94.
- 24. Yannas, I.V. "Materials for Skin and Nerve Regeneration; Biologically Active Analogs of the Extracellular Matrix", Chapter 5 in Materials Science and Technology. Medical and Dental Materials, D.F. Williams, vol. ed., Vol 14. pp. 179-208 (1992).
- 25. Yannas, I.V. "To Regenerate an Organ", Chapter One 6(3):30-33 (1992).
- 26. Yannas, I.V. (1995). "Regeneration templates." Chapter 109, pp. 1619-1635, in The Biomedical Engineering Handbook, J.D. Bronzino, editor, CRC Press.
- 27. Yannas, I.V. (1995). "Artificial skin and dermal equivalents." Chapter 134, pp. 2025-2038, in The Biomedical Engineering Handbook, J.D. Bronzino, ed., CRC Press.
- 28. Yannas, I. V. (1996). Regeneration versus Wiederheistellung des verletzten Gewebes: Die biologische Spezifizität bestimmter Analoga der extrazellulären Matrix, in Wundheilung und Wundauflagen, K. M. Sedlarik, H. Lippert, eds., Wissenschaftliche Verlagsgesellschaft mBH, Stuttgart, pp. 194-202.
- 29. Yannas, I.V. (1996). Natural Materials, Chapter 2.7 in Biomaterials Science: An Introduction to Materials in Medicine, B. Ratner, A. Hoffman, J. Lemons and F. Schoen, eds., Academic Press, NY, pp. 84-94.
- 30. Ellis, D. L. and I. V. Yannas (1996). Tissue regeneration by use of analogs of extracellular matrix, Chapter 18 in Human Biomaterials Applications, D. L. Wise et al., eds., Humana Press, Totowa NJ.
- 31. Yannas, I.V. (1997). "In vivo synthesis of tissues and organs." Chapter 13 in Textbook of Tissue Engineering, Lanza, R.P., R.S. Langer and W. L. Chick, eds., R.G. Landes/Academic Press, New York, pp. 169-178.
- 32. Yannas, I.V. (1997). Models of organ regeneration processes induced by templates. In: Bioartificial Organs, A. Prokop, D. Hunkeler and A.D. Cherrington, eds., Ann. N.Y. Acad. Sci., 831:280-293.
- 33. Louie, L., Yannas, I.V. and Spector, M. (1998). Tendon. In: Frontiers of Tissue Engineering, ed. by C.W. Patrick Jr., A.G. Mikos and L.V. McIntire, Chap. III.4. Pergamon/Elsevier, New York.
- 34. Chamberlain, L. J. and I. V. Yannas (1998). Preparation of collagen-glycosaminoglycan copolymers for tissue regeneration. In J. R. Morgan and M. L. Yarmush, eds. Methods of Molecular Medicine, vol. 18: Tissue Engineering, Humana Press, Tolowa, NJ, Chap. 1, pp. 3-17.
- 35. Landstrom, A. and I. V. Yannas (1999). Peripheral nerve regeneration. In G. Adelman and B. H. Smith, eds. Encyclopedia of Neuroscience, 2nd ed., Elsevier, New York, pp. 1611-1613.
- 36. Yannas, I. V. (1999). In vivo synthesis of organs using collagen-GAG copolymers. In: Tissue Engineering of Vascular Prosthetic Grafts, P. Zilla and H. P. Greisler, Eds. Chap. 52, pp. 571-576. Eds. R. G. Landes, Austin, Texas.
- 37. Yannas I. V. (2000). Facts and models of induced organ regeneration: Skin and peripheral nerve, In H. Garg and M. Longaker, eds. Scarless Healing. Marcel Dekker, New York, pp. 263-277.
- 38. Yannas, I. V. (2000). In vivo synthesis of tissues and organs. In: Lanza, R. P., R. Langer and J. Vacanti, eds., Principles of Tissue Engineering (second edition). Chapter 15, pp. 167-178. Academic Press, New York.
- 39. Yannas, I. V. (2000). Artificial skin and dermal equivalents. In: J. D. Bronzino, ed., The Biomedical Engineering Handbook. Chapter 138, pp.138-1 to 138-15. CRC Press, Boca Raton.
- 40. Yannas, I. V. (2000). Regeneration templates. In: J. D. Bronzino, ed., The Biomedical Engineering Handbook. Chapter 113, pp.113-1 to 113-18. CRC Press, Boca Raton.
- 41. Yannas, I. V. (2000). Natural materials. In: B. Ratner, ed., Second Edition. Biomaterials Science. Academic Press, New York, Boca Raton.
- 42. Harley, B. and I. V. Yannas. 2003. Skin, Tissue Engineering. Chapter in Encyclopedia of Medical Devices, Second Edition, New York, John Wiley and Sons (In the press).
- 43. Brau RR and Yannas IV (2004). Tissue Engineering of Skin. In Encyclopedia of Biomaterials and Biomedical Engineering. pp. 1652-1660. Marcel Dekker.
- 44. Yannas, I. V. 2004. Biologically active scaffolds. In: Scaffolds in Tissue Engineering, ed. J. Elisseef. New York: Humana Press.
- 45. Yannas IV, Wu J and Spilker M. (2004). Peripheral Nerve Regeneration. In Encyclopedia of Neuroscience. 3rd edition. Eds.G. Adelman and B.H. Smith. Elsevier.
Invited Lectures: Available upon request
Theses Supervised by IV Yannas:
S.B. Theses:
- S.A. Kornfeld, The Molecular Basis for Mechanical Deformation of Polyethylene Terephthalate, June 1968.
- D.S. Rogut, A Collagen Engine: Its Design, Construction and Evaluation, June 1968.
- D.S. Mark, Optimizing Collagen Fibers for Use in a Collagen Engine, September 1969.
- B.M. Kinney, Thromboresistivity and Stability of Collagen-Mucopolysaccharide Analogs of Vascular Tissue, May 1976.
- G.D. Speer, Centrifugal Sedimentation Casting of Prosthetic Blood Vessels, May 1976.
- D. Sieverding, Process for Manufacturing Three Dimensional Articles from Collagen, Collagen Mucopolysaccharide Composite, and Other Fibrous Proteins, June 1976.
- R.S. Frank, Manufacture of Collagen and Collagen-Mucopolysaccharide Composite Vessels by Cross Flow Filtration, August 1977.
- R.B. Dobbin, Effects of pH on the Small Angle Diffraction Pattern of Collagen, May 1979.
- R.G. Ackerley, The Effect of Decreasing pH on the Transmission Electron Microscope Image of Collagen, May 1980.
- M.F. Sylvester, Electrical Semiconduction in Collagen, June 1980.
- L.E. Achenie, Electrical Conduction of Halogen Doped Bovine Hide Collagen, May 1981.
- F.T. Thwaites, Manufacture of Collagen-Mucopolysaccharide Vascular Prostheses by Cross Flow Filtration, May 1981.
- C.S. Kang, Siliconizing of an Artificial Skin: Investigation of a Reverse Transfer (Drawdown) Method. June 1981.
- A.M. Sircar, A Biodegradable Adhesive for Medical Applications, June 1982.
- R.W. Wilson, The Effect of Strain and Glycosaminoglycan Content on the Degradation Rate of Collagen-Based Membranes by Bacterial Collagenase, February 1983.
- S.D. Flynn, Effects of Glutaraldehyde Crosslinking and Chondroitin-6-Sulfate upon the Mechanical Properties and In Vivo Healing Response of an Artificial Skin, May 1983.
- P. Kerlee, Mechanical Properties of Skin, May 1983.
- J.F. Kirk, Study of Vapor Induced Crosslinking in Collagen/GAG Foams, May 1983.
- M.L. Paget, A Preliminary Study Using Measurement of Shear Wave Propagation Velocity to Non-Invasively Measure Changes in the Shear Elasticity of Skin Grafts During Healing, January 1984.
- M. Wong, Quantification of Pore Size in Collagen-GAG Artificial Skin, June 1985.
- V.M. Ng, Replacement of Silastic Components of the Artificial Skin with Biodegradable Substitutes, June 1985.
- H. Irving, Effects of Freeze Drying Temperature on the Average Pore Size in Collagen-GAG Artificial Skin, May 1986.
- H.M. Richard III, Replacement of the Silicone Layer of the Artificial Skin with Biodegradable Substitutes, June 1986.
- H. Pickford, Chromium Labeling of an Artificial Skin, June 1986.
- D.A. Gebala, Separation of Epidermal Langerhans Cells, June 1989.
- A. Duros, Development of an In Vitro Model to Study Regeneration of Nerve Cells, May 1991
- C. Raman, C.K., Enzymatic Deletion of Chondroitin 6-Sulfate from Collagene-Glycosaminoglycan Matrices, June 1991.
- N. Sharfman, Processing-Property Relations of Collagen-Glycosaminoglycan Copolymer Foams Freeze-Dried at Various Temperatures, January 1992.
- A. Chong, Wound Healing in an Amphibian Model, 1993.
- [List needs updating. Additional BS theses were completed since 1993.]
S.M. Theses:
- N.K. Jain, Annealing of Internal Stresses in Amorphous Polycarbonate, October 1968.
- S. Arghyros, The Dehydration of Soluble Collagen, January 1969
- J.N. Shah, The Heat Setting of Nylon 6.6, January 1969.
- C. Huang, Solid State Properties of Collagen and Gelatin, January 1971.
- B.L. Lee, Deformation and Recovery of Certain Polymers. An Instrument for the Study of Compressive Creep and Recovery of Swollen Polymers, June 1973.
- C.B. Brogna, Crosslinking Mucopolysaccharides with Divinyl Sulfone, September 1973.
- F.H. Silver, Physicochemical Design and Characterization of Candidate Collagen-Mucopolysaccharide Nonthrombogenic Materials, January l975.
- G.H. Wang, Collagen-Mucopolysaccharide Interaction, September 1975.
- R.H. Rubenstein, Standardization of Collagen-Mucopolysaccharide Composite Materials, February 1977.
- P.G.H. Fernandez, Mechanical Properties and Rate of Enzymatic Degradation of Collagen-Mucopolysaccharide Composite Materials, January 1979.
- P.J. Stasikelis, Burn Dressings Based on Collagen: Structural Parameters Affecting Performance, May 1979.
- M.J. Forbes, Cross-Flow Filtration, Transmission Electron Micrographic Analysis and Blood Compatibility Testing of Collagen Composite Materials for Use as Vascular Prostheses, May 1980.
- D.P. Orgill, Biodegradable Adhesives for Orthopedic Surgery, May 1980.
- M.F. Sylvester, Thrombogenicity of Collagen, February 1982.
- E. H-Y. Chen, The Effects of Porosity and Crosslinking of a Collagen Based Artificial Skin on Wound Healing, June 1982.
- J.F. Kirk, Solid State Crosslinking Process for Collagen-Glycosaminoglycan Membranes, January 1986.
- E. Lee, Effect of Crosslink Density of Collagen Grafts on Wound Contraction Kinetics, May 1986.
- A. S-P. Chang, Electrophysiological Recovery of Peripheral Nerves Regenerated by Biodegradable Polymer Matrix, May 1988.
- H.M. Loree II, A Freeze-Drying Process for Fabrication of Polymeric Bridges for Peripheral Nerve Regeneration, May 1988.
- L.S. Ritterbush, Use of an Extracellular Matrix Analog to Assess Immunogenicity of Epidermal Cell Allografts, February 1992.
- A. Landstrom, Nerve Regeneration Induced by Collagen-GAG Matrix in Collagen Tubes, September 1994.
- L. J. Chamberlain, Long Term Functional and Morphological Evaluation of Peripheral Nerves Regenerated Through Degradable Collagen Implants, September 1996.
- M. Spilker, Studies of Axonal Elongation Across a Gap in the Spinal Cord, January 1997.
- B. Harley. Critical biodegradation rate of tubulation device used in peripheral nerve regeneration. January, 2002.
- R. Brau. Contractile stresses during peripheral nerve regeneration. January, 2002.
- K. Corin. Cancellation of contractile stresses during peripheral nerve regeneration. In progress, 2005.
- E. Soller. Methodology for study of contractile stresses during peripheral nerve regeneration. 2005.
- M. Wong, Genomics of scaffold-induced nerve regeneration. 2006
- A. Sarkar. Effect of upregulation of stem cell presence in skin wound healing. 2007.
- K. Miu. Proteomics of scaffold-induced nerve regeneration. 2009.
- Melissa C. Buydash. Mechanical Modeling of Tissue Response During Early Stage Entubulated Peripheral Nerve Regeneration, 2013.
Doctoral Theses
- A.C. Lunn, The Molecular Basis of Homogeneous Deformation in Glassy Polycarbonate, June 1972.
- N-H. Sung, Structure and Properties of Collagen and Gelatin in the Hydrated and Anhydrous Solid State, June 1972.
- C. Huang, Physicochemical Studies of Collagen and Collagen-Mucopolysaccharide Composite Materials (Model Materials for Skin), February 1974.
- K. Troxel, Mechanisms of Alteration of Skin Wound Contraction Kinetics by Porous Collagen-GAG Materials, December 1994.
- L. Louie, Effect of a Porous Collagen-Glycosaminoglycan Copolymer on Early Tendon Healing in a Novel Animal Model, February 1997.
- L. J. Chamberlain, Influence of Implant Parameters on the Mechanisms of Peripheral Nerve Regeneration, June 1998.
- M.H. Spilker, Peripheral Nerve Regeneration through Tubular Devices. June 2000.
- E. Soller, PhD. Cell-mediated Contraction and Induced Regeneration of the Injured Peripheral Nerve. June 2011.
- D. Tzeranis, PhD. Imaging Studies of Peripheral Nerve Regeneration Induced by Porous Collagen Biomaterials, June 2013.
Patents
Patents and Patent Applications Pending:
- Yannas, J.F. Burke, P.L. Gordon, and C. Huang, "Multilayer Membrane Useful as Synthetic Skin", U.S. Pat. 4,060,081 (November 29, 1977).
- Yannas, P.L. Gordon, C. Huang, F.H. Silver, and J.F. Burke, "Crosslinked Collagen-Mucopolysaccharide Composite Materials", U.S. Pat. 4,280,954 (July 28, 1981).
- Yannas and D.L. Sieverding, "Cross Flow Filtration Molding Method", U.S. Pat. 4,252,759 (February 24, 1981).
- Yannas and M.J. Forbes, "Procedures for Preparing Composite Materials from Collagen and Glycosaminoglycan", U.S. Pat. 4,350,629 (September 21, 1982).
- Yannas, J.F. Burke, D.P. Orgill, and E.M. Skrabut, "Method of Promoting the Regeneration of Tissue at a Wound", U.S. Pat. 4,418,691 (December 6, 1983).
- Yannas and J.F. Kirk, "Method for the Preparation of Collagen-Glycosaminoglycan Composite Materials", U.S. Pat. 4,448,718 (May 15, 1984).
- Yannas and J.F. Burke, "Cell-Seeding Procedures Involving Fibrous Lattices", U.S. Pat. 4,458,678 (July 10, 1984).
- Yannas and J.F. Burke, "Method of Using a Fibrous Lattice", U.S. Pat. 4,505,266 (March 19, 1985).
- Yannas, J.F. Burke, and P.S. Stasikelis, "Method for Preserving Porosity in Porous Materials", U.S. Pat. 4,522,753 (June 11, 1985).
- Yannas, "Process for Forming Multilayer Bioreplaceable Blood Vessel Prosthesis", U.S. Pat. 4,787,900 (November 29, 1988).
- Yannas, "Multilayer Bioreplaceable Blood Vessel Prosthesis", U.S. Pat. 4,902,289 (February 20, 1990).
- Yannas, Elaine Lee, and Ariel Ferdman, "Biodegradable Templates for the Regeneration of Tissues", U.S. Pat. 4,947,840 (August 14, 1990).
- Yannas, D.P. Orgill, H.M. Loree II, J.F. Kirk, A. S.-P. Chang, B.B. Mikic, C. Krarup, T.V. Norregaard, and N.T. Zervas, "Prosthesis for Promotion of Nerve Regeneration", U.S. Pat. 4,955,893 (September 11, 1990).
- Orgill, D. P., C. E. Butler, M. Barlow, S. Ritterbush, I. V. Yannas and C. C. Compton. “Method of skin regeneration using a collagen-glycosaminoglycan matrix and cultured epithelial autograft.”, U.S. Patent 5,489,3041 (1996).
- Orgill D.P., C. E. Butler, M. Barlow, S. Ritterbush, I. V. Yannas, Carolyn C. Compton, “Method of skin regeneration using a collagen-glycosaminoglycan matrix and cultured epithelial autograft”. U.S.Patent 5,716,411 (Feb.10, 1998).
- Spector, M., Yannas, I. V. (and others). "Modification of collagen matrices for prolonged delivery of genes." MIT Case No. 9411. July 25, 2001.
- Gibson, L. J., Yannas, I. V. and others. "Scaffolds with uniform pore channels" (disclosure submitted to MIT). 2003.
- Sannino, A., Yannas I. V. and others. "Novel Technique to Fabricate Porous Tubes with Tube Walls Having Patterned Porosity". U.S. Serial No. 60/622,441. Filed 27 Oct. 2004.
- Yannas I. V., L. J. Gibson, F. J. O’Brien, B. Harley, R. R. Brau, S. Samouhos, M. Spector . “Gradient scaffolding and Methods for Producing the Same". Pub. No. US 2006/0121609 A1 (Patent Application published Jun. 8, 2006)
- Lynn AJ, Yannas IV, B. Harley, L. J. Gibson. “Method for Producing Scaffolds Comprising Porous Layers of Collagen, Calcium Phosphate and GAG”. UK Patent Application No. 0504673.5. (Filed 7 March 2005.)
- Harley BJ, Reddy HK, Yannas IV, Zagorski C. “Processing of angiogenic scaffolds for Large Organ Regeneration”. US Serial no. 60/730,880. Filed 28 Oct. 2005.
- I.V Yannas, H.K. Reddy, C.J. Zagorski, and B.A. Harley, “Gradient Template for Angiogenesis during Large Organ Regeneration”. Intl. application No. PCT/US2006/043424. Intl. Filing Date: Nov. 6, 2006.
- C.J. Zagorski, B.A. Harley, H.K. Reddy, I.V Yannas,. “Tissue Scaffolding Comprising Surface folds for Tissue Engineering”. Intl. Application No. PCT/US2006/14484. Intl. filing Date: April 17, 2006.
- Sannino A, Harley BA, Hastings AZ, Yannas IV; A novel technique to fabricate cylindrical and tubular structures with a patterned porosity. International (PCT) Patent Application patent PCT/US2005/039024. 2005 October 27, 2005.
- Lynn AK, Harley BA, Gibson LJ, Yannas IV, Bonfield W, inventors; Biomaterial. U.K. Patent Application GB05/04673.5. 2005 March 7, 2005.
- M.I.T. Case No. 13111, "Scaffolds for Control of the Fibrotic Healing Response of Liver Tissue", by Karen J. Ho, Seth J. Karp, Eric C. Soller and Ioannis V. Yannas.
- MIT Case No. 13391 Masking of Scaffold Surfaces. Tzeranis D, So P. and Yannas IV.